Core-shell Au@Pd nanoparticles with enhanced catalytic activity for oxygen reduction reaction via core-shell Au@Ag/Pd constructions
- PMID: 26144550
- PMCID: PMC4491719
- DOI: 10.1038/srep11949
Core-shell Au@Pd nanoparticles with enhanced catalytic activity for oxygen reduction reaction via core-shell Au@Ag/Pd constructions
Abstract
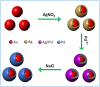
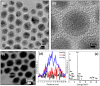
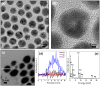
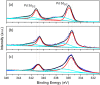
Core-shell nanoparticles often exhibit improved catalytic properties due to the lattice strain created in these core-shell particles. Herein, we demonstrate the synthesis of core-shell Au@Pd nanoparticles from their core-shell Au@Ag/Pd parents. This strategy begins with the preparation of core-shell Au@Ag nanoparticles in an organic solvent. Then, the pure Ag shells are converted into the shells made of Ag/Pd alloy by galvanic replacement reaction between the Ag shells and Pd(2+) precursors. Subsequently, the Ag component is removed from the alloy shell using saturated NaCl solution to form core-shell Au@Pd nanoparticles with an Au core and a Pd shell. In comparison with the core-shell Au@Pd nanoparticles upon directly depositing Pd shell on the Au seeds and commercial Pd/C catalysts, the core-shell Au@Pd nanoparticles via their core-shell Au@Ag/Pd templates display superior activity and durability in catalyzing oxygen reduction reaction, mainly due to the larger lattice tensile effect in Pd shell induced by the Au core and Ag removal.
Figures

References
-
- Bianchini C. & Shen P. K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 109, 4183–4206 (2009). - PubMed
-
- Zhang S., Shao Y., Yin G. & Lin Y. H. Electrostatic self-assembly of a Pt-around-Au nanocomposite with high activity towards formic acid oxidation. Angew. Chem. Int. Ed. 49, 2211–2214 (2010). - PubMed
-
- Uhm S. H., Lee H. J., Kwon Y. K. & Lee J. Y. A stable and cost-effective anode catalyst structure for formic acid fuel cells. Angew. Chem. Int. Ed. 120, 10317–10320 (2008). - PubMed
-
- Xu C., Wang H., Shen P. K. & Jiang S. P. Highly ordered Pd nanowire arrays as effective electrocatalysts for ethanol oxidation in direct alcohol fuel cells. Adv. Mater. 19, 4256–4259 (2007).
-
- Peng Z. & Yang H. PtAu Bimetallic heteronanostructures made by post-synthesis modification of Pt-on-Au nanoparticles. Nano Res. 2, 406–415 (2006).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

