Cardiolipin fingerprinting of leukocytes by MALDI-TOF/MS as a screening tool for Barth syndrome
- PMID: 26144817
- PMCID: PMC4548783
- DOI: 10.1194/jlr.D059824
Cardiolipin fingerprinting of leukocytes by MALDI-TOF/MS as a screening tool for Barth syndrome
Abstract
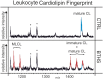
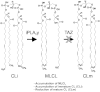
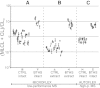
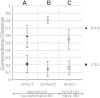
Barth syndrome (BTHS), an X-linked disease associated with cardioskeletal myopathy, neutropenia, and organic aciduria, is characterized by abnormalities of card-iolipin (CL) species in mitochondria. Diagnosis of the disease is often compromised by lack of rapid and widely available diagnostic laboratory tests. The present study describes a new method for BTHS screening based on MALDI-TOF/MS analysis of leukocyte lipids. This generates a "CL fingerprint" and allows quick and simple assay of the relative levels of CL and monolysocardiolipin species in leukocyte total lipid profiles. To validate the method, we used vector algebra to analyze the difference in lipid composition between controls (24 healthy donors) and patients (8 boys affected by BTHS) in the high-mass phospholipid range. The method of lipid analysis described represents an important additional tool for the diagnosis of BTHS and potentially enables therapeutic monitoring of drug targets, which have been shown to ameliorate abnormal CL profiles in cells.
Keywords: cardiomyopathy; lysophospholipids; mass spectrometry; matrix-assisted laser desorption/ionization; mitochondria; phospholipids; phospholipids/metabolism; tafazzin; time-of-flight.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Steward C. G., Martin R. P., Hayes A. M., Salmon A. P., Williams M. M., Tyfield L. A., Davies S. J., Newbury-Ecob R. A. 2004. Barth syndrome (X linked cardiac and skeletal myopathy, neutropenia, and organic aciduria): rarely recognised, frequently fatal [abstract]. Arch. Dis. Child. 89: A48. Abstract G140.
-
- Barth P. G., Scholte H. R., Berden J. A., Van der Klei-Van Moorsel J. M., Luyt-Houwen I. E., Van ’t Veer-Korthof E. T., Van der Harten J. J., Sobotka-Plojhar M. A. 1983. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 62: 327–355. - PubMed
-
- Barth P. G., Van den Bogert C., Bolhuis P. A., Scholte H. R., van Gennip A. H., Schutgens R. B., Ketel A. G. 1996. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): respiratory-chain abnormalities in cultured fibroblasts. J. Inherit. Metab. Dis. 19: 157–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

