Vaccines for women for preventing neonatal tetanus
- PMID: 26144877
- PMCID: PMC7138051
- DOI: 10.1002/14651858.CD002959.pub4
Vaccines for women for preventing neonatal tetanus
Abstract
Background: Tetanus is an acute, often fatal, disease caused by an exotoxin produced by Clostridium tetani. It occurs in newborn infants born to mothers who do not have sufficient circulating antibodies to protect the infant passively, by transplacental transfer. Prevention may be possible by the vaccination of pregnant or non-pregnant women, or both, with tetanus toxoid, and the provision of clean delivery services. Tetanus toxoid consists of a formaldehyde-treated toxin that stimulates the production of antitoxin.
Objectives: To assess the effectiveness of tetanus toxoid, administered to women of reproductive age or pregnant women, to prevent cases of, and deaths from, neonatal tetanus.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2015), CENTRAL (The Cochrane Library 2015, Issue 1), PubMed (1966 to 28 January 2015), EMBASE (1974 to 28 January 2015) and reference lists of retrieved studies.
Selection criteria: Randomised or quasi-randomised trials evaluating the effects of tetanus toxoid in pregnant women or women of reproductive age on numbers of neonatal tetanus cases and deaths.
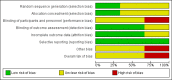
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
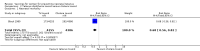
Main results: Two effectiveness trials (9823 infants) and one safety trial (48 mothers) were included. The main outcomes were measured on infants born to a subset of those randomised women who became pregnant during the course of the studies. For our primary outcomes, there was no high-quality evidence according to GRADE assessments.One study (1182 infants) assessed the effectiveness of tetanus toxoid in comparison with influenza vaccine in preventing neonatal tetanus deaths. A single dose did not provide significant protection against neonatal tetanus deaths, (risk ratio (RR) 0.57, 95% confidence interval (CI) 0.26 to 1.24; 494 infants; GRADE: low-quality evidence). However, a two- or three-dose course did provide protection against neonatal deaths, (RR 0.02, 95% CI 0.00 to 0.30; 688 infants; GRADE: moderate-quality evidence). Administration of a two- or three-dose course resulted in significant protection when all causes of death are considered as an outcome (RR 0.31, 95% CI 0.17 to 0.55; 688 infants; GRADE: moderate-quality evidence). No effect was detected on causes of death other than tetanus. Cases of neonatal tetanus after at least one dose of tetanus toxoid were reduced in the tetanus toxoid group, (RR 0.20, 95% CI 0.10 to 0.40; 1182 infants; GRADE: moderate-quality evidence).Another study, involving 8641 children, assessed the effectiveness of tetanus-diphtheria toxoid in comparison with cholera toxoid in preventing neonatal mortality after one or two doses. Neonatal mortality was reduced in the tetanus-diphtheria toxoid group (RR 0.68, 95% CI 0.56 to 0.82). In preventing deaths at four to 14 days, neonatal mortality was reduced again in the tetanus-diphtheria toxoid group (RR 0.38, 95% CI 0.27 to 0.55). The quality of evidence as assessed using GRADE was found to be low.The third small trial assessed that pain at injection site was reported more frequently among pregnant women who received tetanus diphtheria acellular pertussis than placebo (RR 5.68, 95% CI 1.54 to 20.94; GRADE: moderate-quality evidence).
Authors' conclusions: Available evidence supports the implementation of immunisation practices on women of reproductive age or pregnant women in communities with similar, or higher, levels of risk of neonatal tetanus, to the two study sites.
Conflict of interest statement
None known.
Figures








Update of
-
Vaccines for women to prevent neonatal tetanus.Cochrane Database Syst Rev. 2013 May 31;(5):CD002959. doi: 10.1002/14651858.CD002959.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2015 Jul 06;(7):CD002959. doi: 10.1002/14651858.CD002959.pub4. PMID: 23728640 Updated.
References
References to studies included in this review
Black 1980 {published data only}
Munoz 2014 {published data only}
-
- Leibson T, St‐Onge M, Koren G. TDM Journal Club: Safety and immunogenicity of tetanus diphtheria and acellular pertussis immunization during pregnancy. Therapeutic Drug Monitoring 2015;37(3):283‐4. - PubMed
Newell 1966 {published data only}
References to studies excluded from this review
Abu Raya 2014 {published data only}
-
- Abu Raya B, Srugo I, Kessel A, Peterman M, Bader D, Gonen R. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels ‐ A prospective study. Vaccine 2014;32(44):5787‐93. - PubMed
Abuwa 1997 {published data only}
Al‐Safi 2011 {published data only}
-
- Al‐Safi ZA, Shavell VI, Gonik B. Vaccination in pregnancy. Womens Health 2011;7(1):109‐19. - PubMed
Anh 1999 {published data only}
-
- Anh NQ, Hong HA, Nhon TN, Thinh ND, Van NT, Hendriks J. Tetanus antibodies measured by the toxin binding inhibition test (ToBI) in mothers and children in the Neonatal Tetanus Program in Vietnam. Developments in Biological Standardization 1999;101:247‐53. - PubMed
Axelsson 2002 {published data only}
-
- Axelsson I. A Cochrane review on the umbilical cord care and prevention of infections. Antiseptic solutions are not necessary in developed countries but life‐saving in developing countries [Cochrane‐oversikt om att forebygga navelinfektioner. Antiseptisk losning ar onodig i i‐lander men livraddande i u‐lander.]. Lakartidningen 2002;99(14):1563‐6. - PubMed
Aylward 1996 {published data only}
-
- Aylward RB, Mansour E, Oon el‐S A, Tawfik SA, Makar S, Abu el Kheir A, et al. The role of surveillance in a 'high risk' approach to the elimination of neonatal tetanus in Egypt. International Journal of Epidemiology 1996;25(6):1286‐91. - PubMed
Baltazar 1994 {published data only}
-
- Baltazar JC, Sarol JN Jr. Prenatal tetanus immunization and other practices associated with neonatal tetanus. Southeast Asian Journal of Tropical Medicine and Public Health 1994;25(1):132‐8. - PubMed
Basher 2010 {published data only}
-
- Basher MS. Knowledge and practice about TT vaccination among undergraduate female medical students. Mymensingh Medical Journal 2010;19(4):520‐3. - PubMed
Berggren 1971 {published data only}
-
- Berggren WL, Berggren GM. Changing incidence of fatal tetanus of the newborn. A retrospective study in a defined rural Haitian populations. American Journal of Tropical Medicine And Hygiene 1971;20(3):491‐4. - PubMed
Blencowe 2010 {published data only}
Canning 2011 {published data only}
-
- Canning D, Razzaque A, Driessen J, Walker DG, Streatfield PK, Yunus M. The effect of maternal tetanus immunization on children's schooling attainment in Matlab, Bangladesh: follow‐up of a randomized trial. Social Science & Medicine 2011;72(9):1429‐36. - PubMed
Chai 2004 {published data only}
-
- Chai F, Prevots DR, Wang X, Birmingham M, Zhang R, Chai F. Neonatal tetanus incidence in China, 1996‐2001, and risk factors for neonatal tetanus, Guangxi Province, China. International Journal of Epidemiology 2004;33(3):551‐7. - PubMed
Chongsuvivatwong 1993 {published data only}
-
- Chongsuvivatwong V, Bujakorn L, Kanpoy V, Treetrong R. Control of neonatal tetanus in southern Thailand. International Journal of Epidemiology 1993;22(5):931‐5. - PubMed
de Walque 2008 {published data only}
-
- Walque D. Do unsafe tetanus toxoid injections play a significant role in the transmission of HIV/AIDS? Evidence from seven African countries. Sexually Transmitted Infections 2008;84(2):122‐5. - PubMed
Dhillon 1975 {published data only}
-
- Dhillon H, Menon PS. Active immunization of women in pregnancy with two injections of adsorbed tetanus toxoid for prevention of tetanus neonatorum in Punjab, India. Indian Journal of Medical Research 1975;63(4):583‐9. - PubMed
Dietz 1996 {published data only}
Erener‐Ercan 2014 {published data only}
-
- Erener‐Ercan T, Aslan M, Vural M, Erginoz E, Kocazeybek B, Ercan G. Tetanus and diphtheria immunity among term and preterm infant‐mother pairs in Turkey, a country where maternal and neonatal tetanus have recently been eliminated. European Journal of Pediatrics 2015;174(3):339‐44. - PubMed
Gupta 1998 {published data only}
-
- Gupta SD, Keyl PM. Effectiveness of prenatal tetanus toxoid immunization against neonatal tetanus in a rural area in India. Pediatric Infectious Disease Journal 1998;17(4):316‐21. - PubMed
Halperin 2011 {published data only}
-
- Halperin BA, Morris A, Mackinnon‐Cameron D, Mutch J, Langley JM, McNeil SA, et al. Kinetics of the antibody response to tetanus‐diphtheria‐acellular pertussis vaccine in women of childbearing age and postpartum women. Clinical Infectious Diseases 2011;53(9):885‐92. - PubMed
Hardy‐Fairbanks 2013 {published data only}
-
- Hardy‐Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatric Infectious Diseases Journal 2013;32(11):1257‐60. - PubMed
Hasnain 2007 {published data only}
-
- Hasnain S, Sheikh NH. Causes of low tetanus toxoid vaccination coverage in pregnant women in Lahore district, Pakistan. Eastern Mediterranean Health Journal 2007;13(5):1142‐52. - PubMed
Heredia 1968 {published data only}
-
- Heredia AF, Borkar MB, Rao SS. Active immunization in pregnancy with fluid tetanus toxoid. Indian Journal of Medical Sciences 1968;22(4):209‐13. - PubMed
Hlady 1992 {published data only}
Hurmez 2012 {published data only}
-
- Hurmez L, Habeeb QS, Al‐Derzi NA. Seroprevalence of tetanus antibodies among pregnant women in Duhok Governorate, Iraq [Seroprevalence des anticorps antitetaniques chez des femmes enceintes dans le Gouvernorat de Duhok (Iraq)]. Eastern Mediterranean Health Journal 2012;18(6):573‐8. - PubMed
Juan‐Giner 2014 {published data only}
-
- Juan‐Giner A, Domicent C, Langendorf C, Roper MH, Baoundoh P, Fermon F, et al. A cluster randomized non‐inferiority field trial on the immunogenicity and safety of tetanus toxoid vaccine kept in controlled temperature chain compared to cold chain. Vaccine 2014;32(47):6220‐6. - PubMed
Kielmann 1977 {published data only}
-
- Kielmann AA, Vohra SR. Control of tetanus neonatorum in rural communities‐‐immunization effects of high‐dose calcium phosphate‐absorbed tetanus toxoid. Indian Journal of Medical Research 1977;66(6):906‐16. - PubMed
Koenig 1998 {published data only}
Krishnan 2013 {published data only}
-
- Krishnan A, Srivastava R, Dwivedi P, Ng N, Byass P, Pandav CS. Non‐specific sex‐differential effect of DTP vaccination may partially explain the excess girl child mortality in Ballabgarh, India. Tropical Medicine and International Health 2013;18(11):1329‐37. - PubMed
Lassi 2010 {published data only}
MacLennan 1965 {published data only}
Mulholland 1996 {published data only}
-
- Mulholland K, Suara RO, Siber G, Roberton D, Jaffar S, N'Jie J, et al. Maternal immunization with Haemophilus influenzae type b polysaccharide‐tetanus protein conjugate vaccine in the Gambia. JAMA 1996;275(15):1182‐8. - PubMed
Nohynek 1999 {published data only}
-
- Nohynek H, Gustafsson L, Capeding MR, Kayhty H, Olander RM, Pascualk L, et al. Effect of transplacentally acquired tetanus antibodies on the antibody responses to haemophilus influenzae type b‐tetanus toxoid conjugate and tetanus toxoid vaccines in Filipino infants. Pediatric Infectious Disease Journal 1999;18(1):25‐30. - PubMed
Orozova‐Bekkevold 2007 {published data only}
-
- Orozova‐Bekkevold I, Jensen H, Stensballe L, Olsen J. Maternal vaccination and preterm birth: using data mining as a screening tool. Pharmacy World and Science 2007;29(3):205‐12. - PubMed
Perry 1998 {published data only}
Rahman 1982a {published data only}
-
- Rahman M, Chen LC, Chakraborty J, Yunus M, Chowdhury AI, Sarder AM, et al. Use of tetanus toxoid for the prevention of neonatal tetanus. 1. Reduction of neonatal mortality by immunization of non‐pregnant and pregnant women in rural Bangladesh. Bulletin of the World Health Organization 1982;60(2):261‐7. - PMC - PubMed
Rahman 1982b {published data only}
Relyveld 1991 {published data only}
-
- Relyveld E, Bengounia A, Huet M, Kreeftenberg JG. Antibody response of pregnant women to two different absorbed tetanus toxoids. Vaccine 1991;9(5):369‐72. - PubMed
Salama 2009 {published data only}
-
- Salama MM, Hady OA, Ashour W, Mostafa A, Alkamy S, Sayed N, et al. A randomized controlled trial of oral administration of tetanus toxoid (TT) versus tetanus and reduced diphtheria (Td) in pregnant women. Journal of Clinical Immunology 2009;29(4):524‐31. - PubMed
Schofield 1961 {published data only}
Shakib 2013 {published data only}
Silveira 1995 {published data only}
Stanfield 1973 {published data only}
-
- Stanfield JP, Gall D, Bracken PM. Single‐dose antenatal tetanus immunisation. Lancet 1973;1(7797):215‐9. - PubMed
Suri 1964 {published data only}
Tall 1991 {published data only}
-
- Tall F, Prazuck T, Roisin A, Sanou J, Nacro B, Traore A, et al. Risk factors for neonatal tetanus in western Burkina Faso. Case‐control study [Facteurs de risque du tetanos neonatal dans l'ouest du Burkina Faso. Etude cas temoin]. Bulletin de la Societe de Pathologie Exotique 1991;84(5 Pt 5):558‐61. - PubMed
Traverso 1991 {published data only}
Yala 1980 {published data only}
-
- Yala PF. Prevention of neonatal tetanus by active immunization of pregnant women in Brazzaville. Practical evalation and a comparison of single‐dose and triple‐dose vaccinations. Bulletin de la Societe de Pathologie Exotique et de ses Fil 1980;73(1):15‐22. - PubMed
Yusuf 1991 {published data only}
-
- Yusuf B, Solter S, Bertsch D, Arnold RB. Impact of a tetanus toxoid immunization mass campaign on neonatal tetanus mortality in Aceh Province, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health 1991;22(3):351‐6. - PubMed
Additional references
Borrow 2006
-
- Borrow R, Balmer P, Roper MH. The immunological basis for immunization series Module 3: Tetanus Update 2006. Immunisation, Vaccines and Biologicals ‐ WHO http://whqlibdoc.who.int/publications/2007/9789241595551_eng.pdf (accessed on May 4th, 2015).
Descombey 1924
-
- Descombey P. L'anatoxine tetanique. Comptes rendus des séances de la Société de biologie et de ses filiales 1924;91:239‐41.
Dietz 1996
GRADE 2014 [Computer program]
-
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jefferson 1996
-
- Jefferson TO, Jefferson VM. The quest for trials on the efficacy of human vaccines. Results of the handsearch of "Vaccine". Vaccine 1996;14:461‐4. - PubMed
Jefferson 1998
-
- Jefferson TO. Vaccine trial data systematically assembled, pooled and disseminated by the Cochrane Collaboration. Vaccine 1998;16:1487‐95. - PubMed
Liu 2015
-
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post‐2015 priorities: an updated systematic analysis. Lancet 2015;385(9966):430‐40. - PubMed
Prevots 1998
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
-
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. - PubMed
UNICEF/WHO/UNFPA 2000
-
- UNICEF, WHO, UNFPA. Maternal and Neonatal TetanusElimination by 2005. Strategies for achieving and maintaining elimination. http://www.unfpa.org/sites/default/files/pub‐pdf/maternal_health_2000.pdf [accessed April 29th, 2015].
UNICEF/WHO/UNFPA 2015
-
- UNICEF, WHO, UNFPA. Achieving and Sustaining Maternal and NeonatalTetanus Elimination. Strategic Plan 2012–2015. http://www.who.int/immunization/diseases/MNTEStrategicPlan_E.pdf [accessed on April 29th, 2015].
WHO 1999
-
- World Health Organization. Progress towards the global elimination of neonatal tetanus, 1990‐1998. Weekly Epidemiological Record 1999;74(10):73‐80. - PubMed
WHO 2006
-
- World Health Organization. Tetanus vaccine. WHO position paper. Weekly Epidemiological Record 2006;81(20):198‐208.
WHO 2015
-
- World Health Organization. Maternal and Neonatal Tetanus (MNT) elimination. http://www.who.int/immunization/diseases/MNTE_initiative/en/ (accessed April 29th, 2015).
References to other published versions of this review
Demicheli 2001
Demicheli 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

