Corneal structure and transparency
- PMID: 26145225
- PMCID: PMC4655862
- DOI: 10.1016/j.preteyeres.2015.07.001
Corneal structure and transparency
Abstract
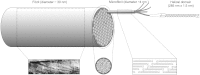
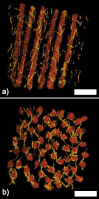
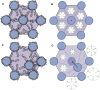
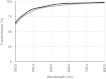
The corneal stroma plays several pivotal roles within the eye. Optically, it is the main refracting lens and thus has to combine almost perfect transmission of visible light with precise shape, in order to focus incoming light. Furthermore, mechanically it has to be extremely tough to protect the inner contents of the eye. These functions are governed by its structure at all hierarchical levels. The basic principles of corneal structure and transparency have been known for some time, but in recent years X-ray scattering and other methods have revealed that the details of this structure are far more complex than previously thought and that the intricacy of the arrangement of the collagenous lamellae provides the shape and the mechanical properties of the tissue. At the molecular level, modern technologies and theoretical modelling have started to explain exactly how the collagen fibrils are arranged within the stromal lamellae and how proteoglycans maintain this ultrastructure. In this review we describe the current state of knowledge about the three-dimensional stromal architecture at the microscopic level, and about the control mechanisms at the nanoscopic level that lead to optical transparency.
Keywords: Collagen; Cornea; Structure; Theoretical modelling; Three-dimensional electron microscopy; Transparency; X-ray.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures









References
-
- Abahussin M., Hayes S., Knox Cartwright N.E., Kamma Lorger C.S., Khan Y., Marshall J., Meek K.M. 3D collagen orientation study in human cornea using X-ray diffraction and femtosecond laser technology. Invest. Ophthalmol. Vis. Sci. 2009;50:5159–5164. - PubMed
-
- Aghamohammadzadeh H., Newton R.H., Meek K.M. X-ray scattering used to map the preferred collagen orientation in the human cornea and limbus. Structure. 2004;12:249–256. - PubMed
-
- Alexander R.A., Garner A. Elastic and precursor fibres in the normal human eye. Exp. Eye Res. 1983;36:305–315. - PubMed
-
- Alexander R.J., Silverman B., Henley W.L. Isolation and characterization of BCP-54, the major soluble protein of bovine cornea. Exp. Eye Res. 1981;32:205–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

