Hypoxia induces calpain activity and degrades SMAD2 to attenuate TGFβ signaling in macrophages
- PMID: 26146544
- PMCID: PMC4491253
- DOI: 10.1186/s13578-015-0026-x
Hypoxia induces calpain activity and degrades SMAD2 to attenuate TGFβ signaling in macrophages
Abstract
Background: Under inflammatory conditions or during tumor progression macrophages acquire distinct phenotypes, with factors of the microenvironment such as hypoxia and transforming growth factor β (TGFβ) shaping their functional plasticity. TGFβ is among the factors causing alternative macrophage activation, which contributes to tissue regeneration and thus, resolution of inflammation but may also provoke tumor progression. However, the signal crosstalk between TGFβ and hypoxia is ill defined.
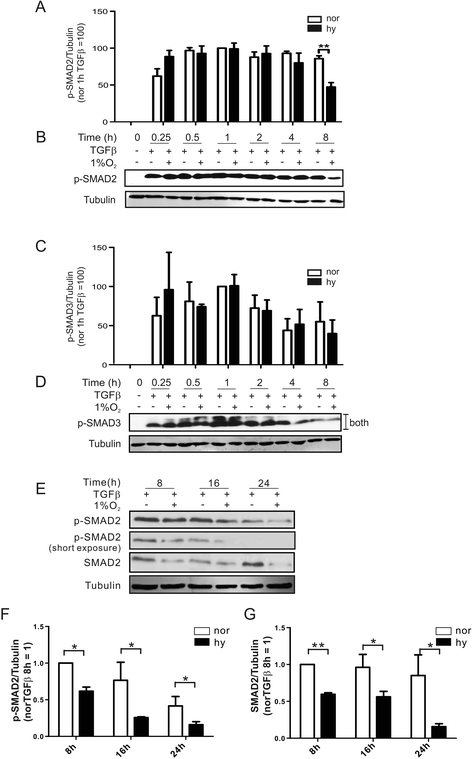
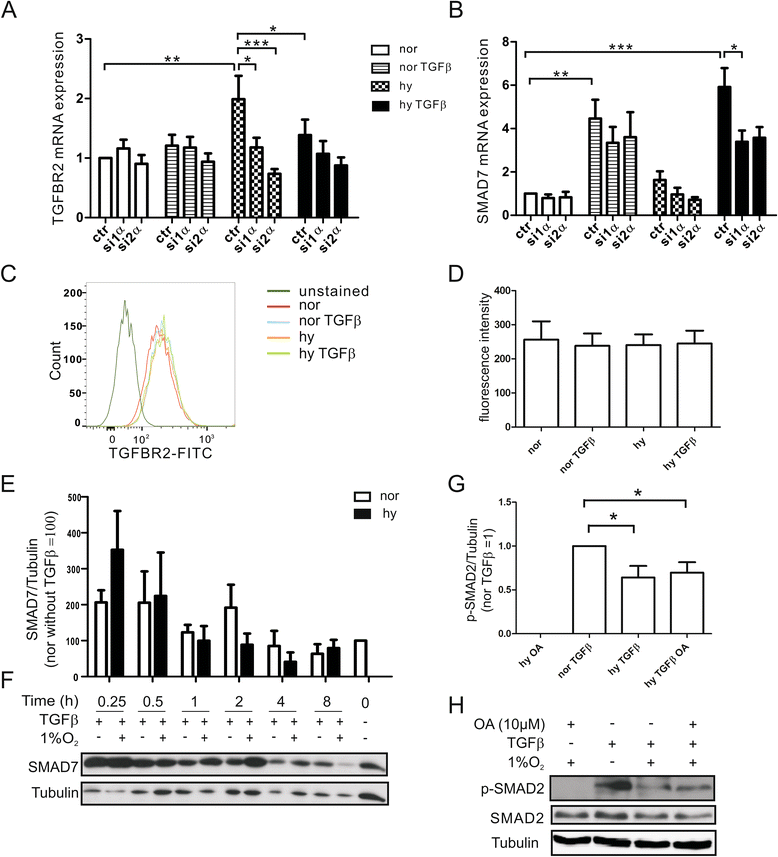
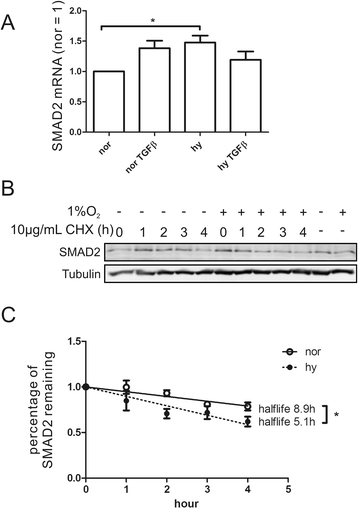
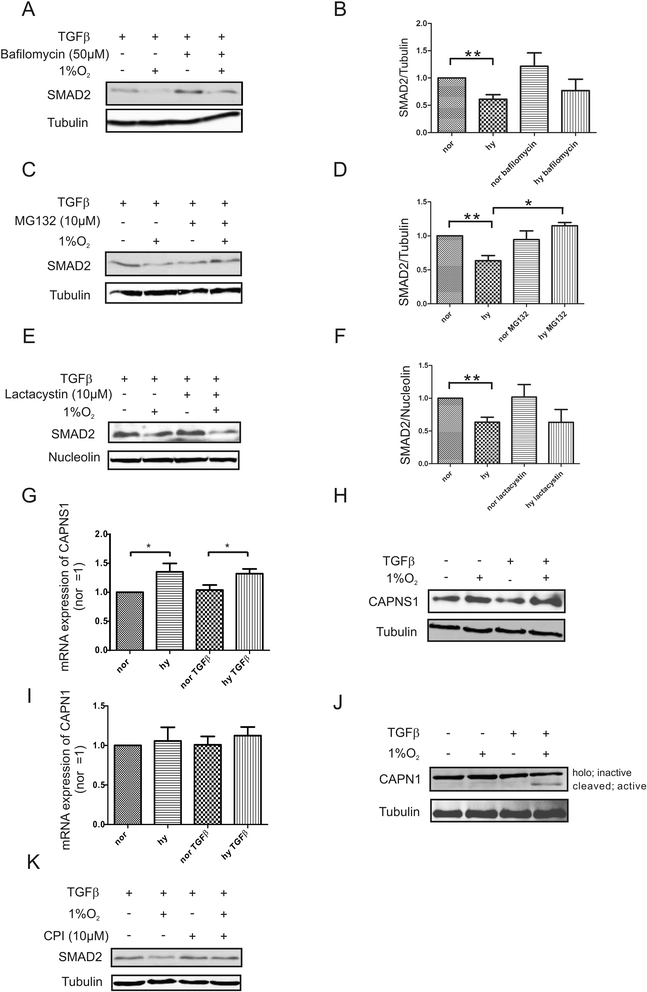
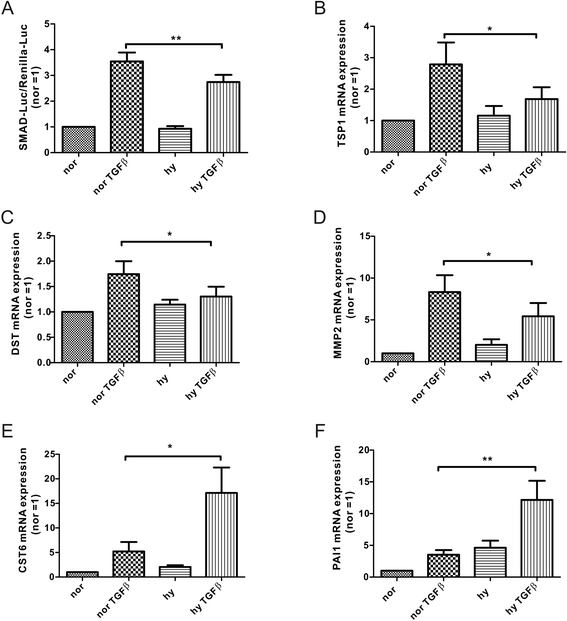
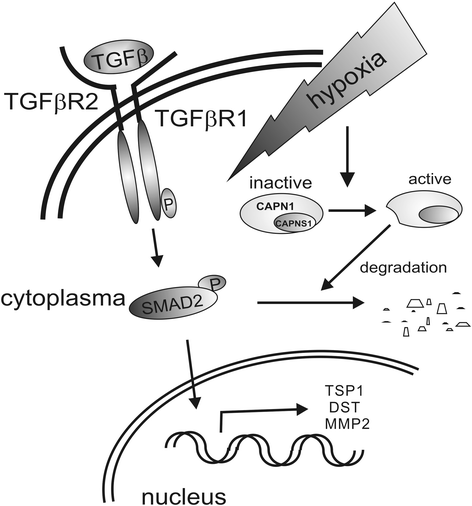
Results: Exposing human primary macrophages to TGFβ elicited a rapid SMAD2/SMAD3 phosphorylation. This early TGFβ-signaling remained unaffected by hypoxia. However, with prolonged exposure periods to TGFβ/hypoxia the expression of SMAD2 declined because of decreased protein stability. In parallel, hypoxia increased mRNA and protein amount of the calpain regulatory subunit, with the further notion that TGFβ/hypoxia elicited calpain activation. The dual specific proteasome/calpain inhibitor MG132 and the specific calpain inhibitor 1 rescued SMAD2 degradation, substantiating the ability of calpain to degrade SMAD2. Decreased SMAD2 expression reduced TGFβ transcriptional activity of its target genes thrombospondin 1, dystonin, and matrix metalloproteinase 2.
Conclusions: Hypoxia interferes with TGFβ signaling in macrophages by calpain-mediated proteolysis of the central signaling component SMAD2.
Keywords: Calpain; Hypoxia; Macrophages; SMAD2 degradation; TGFß; phospho-SMAD2.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

