Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis
- PMID: 26147154
- PMCID: PMC5049673
- DOI: 10.1002/hep.27972
Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis
Abstract
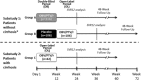
GIFT-I is a phase 3 trial evaluating the efficacy and safety of a 12-week regimen of coformulated ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) for treatment of Japanese hepatitis C virus genotype 1b-infected patients. It consists of a double-blind, placebo-controlled substudy of patients without cirrhosis and an open-label substudy of patients with compensated cirrhosis. Patients without cirrhosis were randomized 2:1 to once-daily OBV/PTV/r (25 mg/150 mg/100 mg; group A) or placebo (group B). Patients with cirrhosis received open-label OBV/PTV/r (group C). The primary efficacy endpoint was the rate of sustained virological response 12 weeks posttreatment in interferon-eligible, treatment-naive patients without cirrhosis and hepatitis C virus RNA ≥100,000 IU/mL in group A. A total of 321 patients without cirrhosis were randomized and dosed with double-blind study drug (106 received double-blind placebo and later received open-label OBV/PTV/r), and 42 patients with cirrhosis were enrolled and dosed with open-label OBV/PTV/r. In the primary efficacy population, the rate of sustained virological response 12 weeks posttreatment was 94.6% (106/112, 95% confidence interval 90.5-98.8). Sustained virological response 12 weeks posttreatment rates were 94.9% (204/215) in group A, 98.1% (104/106) in group B (open-label), and 90.5% (38/42) in group C. Overall, virological failure occurred in 3.0% (11/363) of patients who received OBV/PTV/r. The rate of discontinuation due to adverse events was 0%-2.4% in the three patient groups receiving OBV/PTV/r. The most frequent adverse event in patients in any group was nasopharyngitis.
Conclusion: In this broad hepatitis C virus genotype 1b-infected Japanese patient population with or without cirrhosis, treatment with OBV/PTV/r for 12 weeks was highly effective and demonstrated a favorable safety profile.
© 2015 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures

References
-
- Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology 2010;53:39‐43. - PubMed
-
- Tanaka J, Kumagai J, Katayama K, Komiya Y, Mizui M, Yamanaka R, et al. Sex‐ and age‐specific carriers of hepatitis B and C viruses in Japan estimated by the prevalence in the 3,485,648 first‐time blood donors during 1995‐2000. Intervirology 2004;47:32‐40. - PubMed
-
- Takada A, Tsutsumi M, Okanoue T, Matsushima T, Komatsu M, Fujiyama S. Distribution of the different subtypes of hepatitis C virus in Japan and the effects of interferon: a nationwide survey. J Gastroenterol Hepatol 1996;11:201‐207. - PubMed
-
- Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, et al. Simeprevir with peginterferon/ribavirin for treatment‐naive hepatitis C genotype 1 patients in Japan: CONCERTO‐1, a phase III trial. J Hepatol 2014;61:219‐227. - PubMed
-
- Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, et al. Once‐daily simeprevir with peginterferon and ribavirin for treatment‐experienced HCV genotype 1‐infected patients in Japan: the CONCERTO‐2 and CONCERTO‐3 studies. J Gastroenterol 2014;49:941‐953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
