How to Establish and Follow up a Large Prospective Cohort Study in the 21st Century--Lessons from UK COSMOS
- PMID: 26147611
- PMCID: PMC4492973
- DOI: 10.1371/journal.pone.0131521
How to Establish and Follow up a Large Prospective Cohort Study in the 21st Century--Lessons from UK COSMOS
Abstract
Large-scale prospective cohort studies are invaluable in epidemiology, but they are increasingly difficult and costly to establish and follow-up. More efficient methods for recruitment, data collection and follow-up are essential if such studies are to remain feasible with limited public and research funds. Here, we discuss how these challenges were addressed in the UK COSMOS cohort study where fixed budget and limited time frame necessitated new approaches to consent and recruitment between 2009-2012. Web-based e-consent and data collection should be considered in large scale observational studies, as they offer a streamlined experience which benefits both participants and researchers and save costs. Commercial providers of register and marketing data, smartphones, apps, email, social media, and the internet offer innovative possibilities for identifying, recruiting and following up cohorts. Using examples from UK COSMOS, this article sets out the dos and don'ts for today's cohort studies and provides a guide on how best to take advantage of new technologies and innovative methods to simplify logistics and minimise costs. Thus a more streamlined experience to the benefit of both research participants and researchers becomes achievable.
Conflict of interest statement
Figures
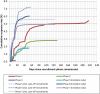
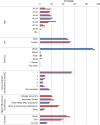
References
-
- Ward H, Toledano MB, Shaddick G, Davies B, Elliott P. Oxford Handbook of Epidemiology for Clinicians. Oxford: Oxford University Press; 2012.
-
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–56. - PubMed
-
- The Nurses' Health Study. History webpage. 2014. Available from: http://www.channing.harvard.edu/nhs/?page_id=70.
-
- Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, et al. UK Biobank: Current status and what it means for epidemiology. Health Policy and Technology. 2012;1(3):123–6.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

