Regulatory T cells and minimal change nephropathy: in the midst of a complex network
- PMID: 26147676
- PMCID: PMC4711159
- DOI: 10.1111/cei.12675
Regulatory T cells and minimal change nephropathy: in the midst of a complex network
Abstract
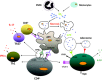
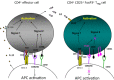
Minimal change nephrosis (MCN) is an important cause of morbidity in children. In spite of successful therapies having been developed in the last three decades, most aspects related to pathogenesis still remain poorly defined. Evolution in basic immunology and results deriving from animal models of the disease suggest a complex interaction of factors and cells starting from activation of innate immunity and continuing with antigen presentation. Oxidants, CD80 and CD40/CD40L have probably a relevant role at the start. Studies in animal models and in human beings also suggest the possibility that the same molecules (i.e. CD80, CD40) are expressed by podocytes under inflammatory stimuli, representing a direct potential mechanism for proteinuria. B and T cells could play a relevant role this contest. Implication of B cells is suggested indirectly by studies utilizing anti-CD20 monoclonal antibodies as the main therapy. The role of regulatory T cells (Tregs ) is supported mainly by results in animal models of nephrotic syndrome (i.e. adriamycin, puromycin, lipopolysaccharide), showing a protective effect of direct Treg infusion or stimulation by interleukin 2 (IL-2). Limited studies have also shown reduced amounts of circulating Tregs in patients with active MCN cells. The route from bench to bedside would be reduced if results from animal models were confirmed in human pathology. The expansion of Tregs with recombinant IL-2 and new anti-CD20 monoclonal antibodies is the beginning. Blocking antigen-presenting cells with cytotoxic T lymphocyte antigen (CTLA-4)-Ig fusion molecules inhibiting CD80 and/or with blockers of CD40-CD40 ligand interaction represent potential new approaches. The hope is that evolution in therapies of MCN could fill a gap lasting 30 years.
Keywords: IL-2; LPS nephropathy; minimal change nephropathy; regulatory T cells.
© 2015 British Society for Immunology.
Figures

References
-
- Kidney Disease Improving Global Outcomes (KDIGO) . Clinical practice guideline for glomerulonephritis. Kidney Int 2012; 2:181–5.
-
- Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long‐term outcome for children with minimal‐change nephrotic syndrome. Lancet 1985; 1:368–70. - PubMed
-
- Ponticelli C, Rizzoni G, Edefonti A et al A randomized trial of cyclosporine in steroid‐resistant idiopathic nephrotic syndrome. Kidney Int 1993; 43:1377–84. - PubMed
-
- Ghiggeri GM, Catarsi P, Scolari F et al Cyclosporine in patients with steroid‐resistant nephrotic syndrome: an open‐label, nonrandomized, retrospective study. Clin Ther 2004; 26:1411–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

