iStent with Phacoemulsification versus Phacoemulsification Alone for Patients with Glaucoma and Cataract: A Meta-Analysis
- PMID: 26147908
- PMCID: PMC4492499
- DOI: 10.1371/journal.pone.0131770
iStent with Phacoemulsification versus Phacoemulsification Alone for Patients with Glaucoma and Cataract: A Meta-Analysis
Abstract
Background: Minimally invasive glaucoma surgeries (MIGS) have attracted significant attention, as they have been reported to lower intra-ocular pressure (IOP) and have an excellent safety profile. The iStent is an example of a minimally invasive glaucoma device that has received particular attention due to its early and wide spread utilization. There is a growing body of evidence supporting its use at the time of phacoemulsification to help lower IOP. However, it is still not clear how much of the IOP lowering effect can be attributed to the iStent, the crystalline lens extraction or both when inserted concurrently at the time of phacoemulsification. This has been an important issue in understanding its potential role in the glaucoma management paradigm.
Purpose: To conduct a systematic review and meta-analysis comparing the IOP lowering effect of iStent insertion at the time of phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataracts.
Methods: A systematic review was conducted utilizing various databases. Studies examining the IOP lowering effect of iStent insertion in combination with phacoemulsification, as well as studies examining the IOP lowering effect of phacoemulsification alone were included. Thirty-seven studies, reporting on 2495 patients, met the inclusion criteria. The percentage reduction in IOP (IOPR%) and mean reduction in topical glaucoma medications after surgery were determined. The standardized mean difference (SMD) was computed as a measure of the treatment effect for continuous outcomes taking into account heterogeneity. Fixed-effect and random-effect models were applied.
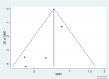
Results: A 4% IOP reduction (IOPR%) from baseline occurred following phacoemulsification as a solo procedure compared to 9% following an iStent implant with phacoemulsification, and 27% following 2 iStents implants with phacoemulsification. Compared with cataract extraction alone, iStent with phacoemulsification resulted in significant reduction in the post-operative IOP (IOPR%) (SMD = -0.46, 95% CI: [-0.87, -0.06]). A weighted mean reduction in the number of glaucoma medications per patient was 1.01 following phacoemulsification alone compared to 1.33 after one iStent implant with phacoemulsification, and 1.1 after 2 iStent implants with phacoemulsification. Compared to cataract extraction alone, iStent with cataract extraction showed a significant decrease in the number of glaucoma medications (SMD = -0.65, 95% CI: [-1.18, -0.12]). Funnel plots suggested the absence of publication bias.
Conclusion: Both iStent implantation with concurrent phacoemulsification and phacoemulsification alone result in a significant decrease in IOP and topical glaucoma medications. In terms of both reductions, iStent with phacoemulsification significantly outperforms phacoemulsification alone.
Conflict of interest statement
Figures







References
-
- The National Coalition for Vision Health (2010) Vision Loss in Canada 2011.
-
- Prevent Blindness America (2013) Cost of Vision Problems: The Economic Burden of Vision Loss and Eye Disorders in the United States National Opinion Research Center, University of Chicago.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

