Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age
- PMID: 26147946
- PMCID: PMC4570860
- DOI: 10.1001/jamaneurol.2015.1285
Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age
Abstract
Importance: Individuals in the presymptomatic stage of Alzheimer disease (AD) are increasingly being targeted for AD secondary prevention trials. How early during the normal life span underlying AD pathologies begin to develop, their patterns of change over time, and their relationship with future cognitive decline remain to be determined.
Objective: To characterize the within-person trajectories of cerebrospinal fluid (CSF) biomarkers of AD over time and their association with changes in brain amyloid deposition and cognitive decline in cognitively normal middle-aged individuals.
Design, setting, and participants: As part of a cohort study, cognitively normal (Clinical Dementia Rating [CDR] of 0) middle-aged research volunteers (n = 169) enrolled in the Adult Children Study at Washington University, St Louis, Missouri, had undergone serial CSF collection and longitudinal clinical assessment (mean, 6 years; range, 0.91-11.3 years) at 3-year intervals at the time of analysis, between January 2003 and November 2013. A subset (n = 74) had also undergone longitudinal amyloid positron emission tomographic imaging with Pittsburgh compound B (PiB) in the same period. Serial CSF samples were analyzed for β-amyloid 40 (Aβ40), Aβ42, total tau, tau phosphorylated at threonine 181 (P-tau181), visinin-like protein 1 (VILIP-1), and chitinase-3-like protein 1 (YKL-40). Within-person measures were plotted according to age and AD risk defined by APOE genotype (ε4 carriers vs noncarriers). Linear mixed models were used to compare estimated biomarker slopes among middle-age bins at baseline (early, 45-54 years; mid, 55-64 years; late, 65-74 years) and between risk groups. Within-person changes in CSF biomarkers were also compared with changes in cortical PiB binding and progression to a CDR higher than 0 at follow-up.
Main outcomes and measures: Changes in Aβ40, Aβ42, total tau, P-tau181, VILIP-1, and YKL-40 and, in a subset of participants, changes in cortical PiB binding.
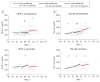
Results: While there were no consistent longitudinal patterns in Aβ40 (P = .001-.97), longitudinal reductions in Aβ42 were observed in some individuals as early as early middle age (P ≤ .05) and low Aβ42 levels were associated with the development of cortical PiB-positive amyloid plaques (area under receiver operating characteristic curve = 0.9352; 95% CI, 0.8895-0.9808), especially in mid middle age (P < .001). Markers of neuronal injury (total tau, P-tau181, and VILIP-1) dramatically increased in some individuals in mid and late middle age (P ≤ .02), whereas the neuroinflammation marker YKL-40 increased consistently throughout middle age (P ≤ .003). These patterns were more apparent in at-risk ε4 carriers (Aβ42 in an allele dose-dependent manner) and appeared to be associated with future cognitive deficits as determined by CDR.
Conclusions and relevance: Longitudinal CSF biomarker patterns consistent with AD are first detectable during early middle age and are associated with later amyloid positivity and cognitive decline. Such measures may be useful for targeting middle-aged, asymptomatic individuals for therapeutic trials designed to prevent cognitive decline.
Conflict of interest statement
Figures


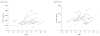

Comment in
-
Alzheimer disease: Cerebrospinal fluid markers for AD change during middle age.Nat Rev Neurol. 2015 Aug;11(8):427. doi: 10.1038/nrneurol.2015.129. Epub 2015 Jul 28. Nat Rev Neurol. 2015. PMID: 26215625 No abstract available.
References
-
- Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 suppl):S85–S88. - PubMed
-
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57(12):1168–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

