Milnacipran for neuropathic pain in adults
- PMID: 26148202
- PMCID: PMC6485877
- DOI: 10.1002/14651858.CD011789
Milnacipran for neuropathic pain in adults
Abstract
Background: Milnacipran is a serotonin-norepinephrine reuptake inhibitor (SNRI) that is sometimes used to treat chronic neuropathic pain and fibromyalgia. This is an update of an earlier review of milnacipran for neuropathic pain and fibromyalgia in adults originally published in The Cochrane Library Issue 3, 2012. We split that review so that this one looked only at neuropathic pain, and a separate review looks at fibromyalgia.
Objectives: To assess the analgesic efficacy and associated adverse events of milnacipran for chronic neuropathic pain in adults.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and EMBASE to 23 February 2015, together with reference lists of retrieved papers and reviews.
Selection criteria: We included randomised, double-blind studies of eight weeks' duration or longer, comparing milnacipran with placebo or another active treatment in chronic neuropathic pain.
Data collection and analysis: Two review authors independently searched for studies, extracted efficacy and adverse event data, and examined issues of study quality. We did not carry out any analysis.
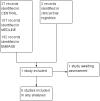
Main results: We included a single study of 40 participants with chronic low back pain with a neuropathic component. It found no difference in pain scores between milnacipran 100 mg to 200 mg daily or placebo after six weeks (very low quality evidence). Adverse event rates were similar between treatments, with too few data to draw conclusions (very low quality evidence).
Authors' conclusions: There was no evidence to support the use of milnacipran to treat neuropathic pain conditions.
Conflict of interest statement
SD has no conflicts relating to this review or any similar product.
TP has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
PJW has no conflicts relating to this review or any similar product.
For transparency we have received research support from charities, government, and industry sources at various times, but none relate to this review. We are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
References
References to studies included in this review
NCT01225068 {published data only}
-
- An exploratory randomized placebo controlled trial of milnacipran in patients with chronic neuropathic low back pain. clinicaltrials.gov/ct2/show/record/NCT01225068 (accessed 23 February 2015). [CTG: NCT01225068]
References to ongoing studies
NCT01288937 {published data only}
-
- A placebo controlled, randomized, double blind trial of milnacipran for the treatment of idiopathic neuropathy pain. clinicaltrials.gov/ct2/show/NCT01288937 (accessed 23 February 2015). [CTG: NCT01288937]
Additional references
Baron 2010
Baron 2012
Bouhassira 2008
Cording 2015
Dechartres 2013
Derry 2012b
Derry 2013
Derry 2014
Di Franco 2010
-
- Franco M, Iannuccelli C, Atzeni F, Cazzola M, Salaffi F, Valesini G, et al. Pharmacological treatment of fibromyalgia. Clinical and Experimental Rheumatology 2010;28(6 Suppl 63):S110‐6. - PubMed
Dworkin 2008
Finnerup 2015
Gustorff 2008
-
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self‐reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiologica Scandinavica 2008;52(1):132‐6. - PubMed
Hall 2008
Hall 2013
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2010
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] - PubMed
Jensen 2011
Kalso 2013
Katusic 1991
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Koopman 2009
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Lunn 2014
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2]
McQuay 2007
-
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raferty J, Mant J, Simpson S editor(s). Health Care Needs Assessment. Oxford: Radcliffe Publishing Ltd, 2007:519‐600. [ISBN: 978‐1‐84619‐063‐6]
Moisset 2007
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009
Moore 2010a
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] - DOI - PubMed
Moore 2010b
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010c
Moore 2010d
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: ] - PubMed
Moore 2011b
Moore 2012a
Moore 2012b
Moore 2013a
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2014c
Moulin 2014
NICE 2013
-
- National Institute for Health and Care Excellence (NICE). Neuropathic pain ‐ pharmacological management: the pharmacological management of neuropathic pain in adults in non‐specialist settings, 2013. www.nice.org.uk/guidance/cg173 (accessed 23 February 2015).
Nüesch 2010
O'Brien 2010
O'Connor 2009
Pae 2009
PaPaS 2012
-
- PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 23 February 2015).
Rappaport 1994
-
- Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self‐sustaining discharge in the trigeminal ganglion. Pain 1994;56:127‐38. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saarto 2007
Soni 2013
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Suzuki 2004
-
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5‐HT pathways that control spinal pain processing. Trends in the Pharmacological Sciences 2004;2512:613‐7. - PubMed
Torrance 2006
Tracey 2011
Treede 2008
van Hecke 2014
Vo 2009
von Hehn 2012
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical