Luteal phase support for assisted reproduction cycles
- PMID: 26148507
- PMCID: PMC6461197
- DOI: 10.1002/14651858.CD009154.pub3
Luteal phase support for assisted reproduction cycles
Abstract
Background: Progesterone prepares the endometrium for pregnancy by stimulating proliferation in response to human chorionic gonadotropin(hCG) produced by the corpus luteum. This occurs in the luteal phase of the menstrual cycle. In assisted reproduction techniques(ART), progesterone and/or hCG levels are low, so the luteal phase is supported with progesterone, hCG or gonadotropin-releasing hormone (GnRH) agonists to improve implantation and pregnancy rates.
Objectives: To determine the relative effectiveness and safety of methods of luteal phase support provided to subfertile women undergoing assisted reproduction.
Search methods: We searched databases including the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO and trial registers. We conducted searches in November 2014, and further searches on 4 August 2015.
Selection criteria: Randomised controlled trials (RCTs) of luteal phase support using progesterone, hCG or GnRH agonist supplementation in ART cycles.
Data collection and analysis: Three review authors independently selected trials, extracted data and assessed risk of bias. We calculated odds ratios (ORs) and 95%confidence intervals (CIs) for each comparison and combined data when appropriate using a fixed-effect model. Our primary out come was live birth or ongoing pregnancy. The overall quality of the evidence was assessed using GRADE methods.
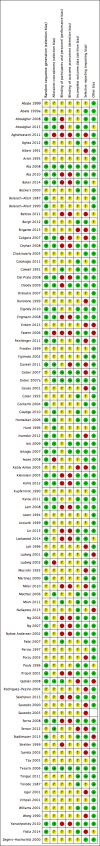
Main results: Ninety-four women RCTs (26,198 women) were included. Most studies had unclear or high risk of bias in most domains. The main limitations in the evidence were poor reporting of study methods and imprecision due to small sample sizes.1. hCG vs placebo/no treatment (five RCTs, 746 women)There was no evidence of differences between groups in live birth or ongoing pregnancy (OR 1.67, 95% CI 0.90 to 3.12, three RCTs,527 women, I2 = 24%, very low-quality evidence, but I2 of 61% was found for the subgroup of ongoing pregnancy) with a random effects model. hCG increased the risk of ovarian hyperstimulation syndrome (OHSS) (1 RCT, OR 4.28, 95% CI 1.91 to 9.6, low quality evidence).2. Progesterone vs placebo/no treatment (eight RCTs, 875 women)Evidence suggests a higher rate of live birth or ongoing pregnancy in the progesterone group (OR 1.77, 95% CI 1.09 to 2.86, five RCTs, 642 women, I2 = 35%, very low-quality evidence). OHSS was not reported.3. Progesterone vs hCG regimens (16 RCTs, 2162 women)hCG regimens included comparisons of progesterone versus hCG and progesterone versus progesterone + hCG. No evidence showed differences between groups in live birth or ongoing pregnancy (OR 0.95, 95% CI 0.65 to 1.38, five RCTs, 833 women, I2 = 0%, low quality evidence) or in the risk of OHSS (four RCTs, 615 women, progesterone vs hCG OR 0.54, 95% CI 0.22 to 1.34; four RCTs,678 women; progesterone vs progesterone plus hCG, OR 0.34, 95% CI 0.09 to 1.26, low-quality evidence).4. Progesterone vs progesterone with oestrogen (16 RCTs, 2577 women)No evidence was found of differences between groups in live birth or ongoing pregnancy (OR 1.12, 95% CI 0.91 to 1.38, nine RCTs,1651 women, I2 = 0%, low-quality evidence) or OHSS (OR 0.56, 95% CI 0.2 to 1.63, two RCTs, 461 women, I2 = 0%, low-quality evidence).5. Progesterone vs progesterone + GnRH agonist (seven RCTs, 1708 women)Live birth or ongoing pregnancy rates were lower in the progesterone-only group and increased in women who received progester one and one or more GnRH agonist doses (OR 0.62, 95% CI 0.48 to 0.81, nine RCTs, 2861 women, I2 = 55%, random effects, low quality evidence). Statistical heterogeneity for this comparison was high because of unexplained variation in the effect size, but the direction of effect was consistent across studies. OHSS was reported in one study only (OR 1.00, 95% CI 0.33 to 3.01, 1 RCT, 300 women, very low quality evidence).6. Progesterone regimens (45 RCTs, 13,814 women)The included studies reported nine different comparisons between progesterone regimens. Findings for live birth or ongoing pregnancy were as follows: intramuscular (IM) versus oral: OR 0.71, 95% CI 0.14 to 3.66 (one RCT, 40 women, very low-quality evidence);IM versus vaginal/rectal: OR 1.24, 95% CI 1.03 to 1.5 (seven RCTs, 2309 women, I2 = 71%, very low-quality evidence); vaginal/rectal versus oral: OR 1.19, 95% CI 0.83 to 1.69 (four RCTs, 857 women, I2 = 32%, low-quality evidence); low-dose versus high-dose vaginal: OR 0.97, 95% CI 0.84 to 1.11 (five RCTs, 3720 women, I2 = 0%, moderate-quality evidence); short versus long protocol:OR 1.04, 95% CI 0.79 to 1.36 (five RCTs, 1205 women, I2 = 0%, low-quality evidence); micronised versus synthetic: OR 0.9, 95%CI 0.53 to 1.55 (two RCTs, 470 women, I2 = 0%, low-quality evidence); vaginal ring versus gel: OR 1.09, 95% CI 0.88 to 1.36 (oneRCT, 1271 women, low-quality evidence); subcutaneous versus vaginal gel: OR 0.92, 95% CI 0.74 to 1.14 (two RCTs, 1465 women,I2 = 0%, low-quality evidence); and vaginal versus rectal: OR 1.28, 95% CI 0.64 to 2.54 (one RCT, 147 women, very low-quality evidence). OHSS rates were reported for only two of these comparisons: IM versus oral, and low versus high-dose vaginal. No evidence showed a difference between groups.7. Progesterone and oestrogen regimens (two RCTs, 1195 women)The included studies compared two different oestrogen protocols. No evidence was found to suggest differences in live birth or ongoing pregnancy rates between a short and a long protocol (OR 1.08, 95% CI 0.81 to 1.43, one RCT, 910 women, low-quality evidence) or between a low dose and a high dose of oestrogen (OR 0.65, 95% CI 0.37 to 1.13, one RCT, 285 women, very low-quality evidence).Neither study reported OHSS.
Authors' conclusions: Both progesterone and hCG during the luteal phase are associated with higher rates of live birth or ongoing pregnancy than placebo.The addition of GnRHa to progesterone is associated with an improvement in pregnancy outcomes. OHSS rates are increased with hCG compared to placebo (only study only). The addition of oestrogen does not seem to improve outcomes. The route of progester one administration is not associated with an improvement in outcomes.
Conflict of interest statement
None.
Figures




















































Update of
-
Luteal phase support for assisted reproduction cycles.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD009154. doi: 10.1002/14651858.CD009154.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Jul 07;(7):CD009154. doi: 10.1002/14651858.CD009154.pub3. PMID: 21975790 Updated.
References
References to studies included in this review
Abate 1999 {published data only}
-
- Abate A, Brigandi A, Abate FG, Manti F, Unfer V, Perino M. Luteal phase support with 17alpha‐hydroxyprogesterone versus unsupported cycles in in vitro fertilization: a comparative randomized study. Gynaecologic and Obstetric Investigation 1999;48(2):78‐80. - PubMed
-
- Abate A, Brigandi A, Costabile L, Abate FG, Balzano E, Perino M. 17‐alpha‐Hydroxyprogesterone caproate and natural progesterone in assisted reproduction: a comparative study. Clinical and Experimental Obstetrics & Gynecology 1997;24(4):190‐2. - PubMed
Abate 1999a {published data only}
-
- Abate A, Perino M, Abate FG, Brigandi A, Costabile L, Manti F. Intramuscular versus vaginal administration of progesterone for luteal phase support after in vitro fertilization and embryo transfer. A comparative randomized study. Clinical and Experimental Obstetrics and Gynecology 1999;26:203‐6. - PubMed
Aboulghar 2008 {published data only}
-
- Aboulghar MA, Amin YM, Al‐Inany HG, Aboulghar MM, Mourad LM, Serour GI, et al. Prospective randomized study comparing luteal phase support for ICSI patients up to the first ultrasound compared with an additional three weeks. Human Reproduction 2008;23(4):857‐62. - PubMed
Aboulghar 2015 {published data only}
-
- Aboulghar, Mohamed A, Marie, Heba, Amin, Yahia M, Aboulghar, Mona M, Nasr, Ahmed, Serour, Gamal I, Mansour, Ragaa T. GnRH agonist plus vaginal progesterone for luteal phase support in ICSI cycles: a randomized study.. Reproductive Biomedicine Online 2015;30:52‐56. - PubMed
Aghahosseini 2011 {published data only}
-
- Aghahosseini M, Aleyassin A, Khodaverdi S, Esfahani F, Mohammadbeigi R, Movahedi S, et al. Estradiol supplementation during the luteal phase in poor responder patients undergoing in vitro fertilization: a randomized clinical trial. Journal of Assisted Reproduction and Genetics 2011;28:785‐90. - PMC - PubMed
Aghsa 2012 {published data only}
-
- Aghsa MM, Rahmanpour H, Bagheri M, Davari‐Tanha F, Nasr R. A randomized comparison of the efficacy, side effects and patient convenience between vaginal and rectal administration of Cyclogest when used for luteal phase support in ICSI treatment. Archives of Gynecology and Obstetrics 2012;286:1049‐54. - PubMed
Albert 1991 {published data only}
-
- Albert J, Pfeifer S. Luteal phase hormone levels after in vitro fertilization and embryo transfer (IVF‐ET): a prospective randomized trial of human chorionic gonadotropin (hCG) vs. intramuscular (im) progesterone (P) for luteal phase support following stimulation with gonadotropin‐releasing hormone agonist (GnRH‐a) and human menopausal gonadotropins (hMG) [abstract]. Fertility and Sterility. 1991:S18 (Abs # O‐041).
Artini 1995 {published data only}
-
- Artini PG, Volpe A, Angioni S, Galassi MC, Battaglia C, Genazzani AR. A comparative, randomized study of three different progesterone support of the luteal phase following IVF/ET program. Journal of Endocrinological Investigation 1995;18:51‐6. - PubMed
Ata 2008 {published data only}
-
- Ata B, Yakin K, Balaban B, Urman B. GnRH agonist protocol administration in the luteal phase in ICSI‐ET cycles stimulated with the long GnRH agonist protocol: a randomized, controlled double blind study. Human Reproduction 2008;23(3):668‐73. - PubMed
-
- Urman B. Single dose gonadotropin‐releasing hormone (GnRH) agonist administration in the luteal phase of GnRH antagonist stimulated ICSI‐ET cycles. Directly obtained from author, 17 March 2011. [Clinicaltrials.gov: NCT01007851]
Ata 2010 {published and unpublished data}
-
- Ata B, Kucuk M, Seyhan A, Urman B. Effect of high‐dose estrogen in luteal phase support on live birth rates after assisted reproduction treatment cycles. Journal of Reproductive Medicine for the Obstetrician and Gynecologist 2010;55:485‐90. - PubMed
Baker 2014 {published and unpublished data}
-
- Baker VL, Jones CA, Doody K, Foulk R, Yee B, Adamson GD, et al. A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for luteal phase support of in vitro fertilization. Human Reproduction 2014;29(10):12‐20. [DOI: 10.1093/humrep/deu194] - DOI - PMC - PubMed
Beckers 2000 {published data only}
-
- Beckers NGM, Laven JSE, Eijkemans MJC, Fauser BCJM. Follicular and luteal phase characteristics following early cessation of gonadotrophin‐releasing hormone agonist during ovarian stimulation for in‐vitro fertilization. Human Reproduction 2000;15(1):43‐9. - PubMed
Belaisch‐Allart 1987 {published data only}
-
- Belaisch‐Allart J, Testart J, Fries N, Forman R G, Frydman R. The effect of dydrogesterone supplementation in an IVF programme. Human Reproduction 1987;2:183‐5. - PubMed
-
- Belaisch‐Allart J, Testart J, Fries N, Forman R, Hazout A, Declerc l, et al. The effect of dydrogesterone and hCG supplementation in an IVF program [abstract]. 5th World Congress on In Vitro Fertilization and Embryo Transfer Abstract Book. 1987:41 (Abs #PP‐35).
Belaisch‐Allart 1990 {published data only}
-
- Belaisch‐Allart J, Mouzon J, Lapousterle C, Mayer M. The effect of HCG supplementation after combined GnRH agonist/HMG treatment in an IVF programme. Human Reproduction 1990;5:163‐6. - PubMed
Beltsos 2011 {published data only}
-
- Beltsos A, Sanchez M, Doody K, Bush M, Scobey J. Efficacy of vaginal progesterone inserts (Endometrin®) compared to intramuscular progesterone in oil for luteal support in PCOS patients. Fertility and Sterility 2011;96(Suppl):S130 (Abs # P‐71).
Bergh 2012 {published data only}
-
- Bergh C, Lindenberg S. A prospective randomized multicentre study comparing vaginal progesterone gel and vaginal micronized progesterone tablets for luteal support after in vitro fertilization/intracytoplasmic sperm injection. Human Reproduction 2012;27(12):3467‐73. - PubMed
Brigante 2013 {published data only}
-
- Brigante CMM, Mignini Renzini M, Dal Canto M, Coticchio G, Comi R, Fadini R. Efficacy of luteal phase support with GnRH agonists: a preliminary comparative study. Fertility and Sterility 2013;100(Suppl):S299 (Abs # P‐521).
Caligara 2007 {published data only}
-
- Caligara C, Carranza F, Ramos J, Rodriguez I, Gonzalez A, Fernandez‐Sanchez M. Luteal phase support in IVF patients at low risk for OHSS: progesterone vs. progesterone plus HCG. A prospective randomized study. Fertility and Sterility 2007;88:S163 (Abs#P‐166).
Ceyhan 2008 {published data only}
-
- Ceyhan ST, Basaran M, Kemal Duru N, Yilmaz A, Goktolga U, Baser I. Use of luteal estrogen supplementation in normal responder patients treated with fixed multidose GnRH antagonist: a prospective randomized controlled study. Fertility and Sterility 2008;89(6):1827‐30. - PubMed
Chakravarty 2005 {published data only}
-
- Chakravarty BN, Shirazee HH, Dam P, Chattopadhyay R, Ghosh S, Dam M, Goswami SK. A randomized prospective study comparing dydrogesterone and intravaginal micronised progesterone as luteal phase support in ART cycle [abstract]. Human Reproduction. 2004; Vol. 19:i126 (Abs #P‐364).
-
- Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study. Journal of Steroid Biochemistry and Molecular Biology 2005;97(5):416‐20. - PubMed
Colakoglu 2011 {published data only}
-
- Colakoglu M, Toy H, Icen MS, Vural M, Mahmoud AS, Yazici F. The impact of estrogen supplementation on IVF outcome in patients with polycystic ovary syndrome. Human Reproduction 2011;26(Suppl 1):i296 (Abs # P‐450).
Colwell 1991 {published data only}
-
- Colwell KA, Tummon IS. Elevation of serum progesterone with oral micronized progesterone after in vitro fertilization. Journal of Reproductive Medicine 1991;36:170‐2. - PubMed
Dal Prato 2008 {published data only}
-
- Dal Prato L, Bianchi L, Cattoli M, Tarozzi N, Flamigni C, Borini A. Vaginal gel versus intramuscular progesterone for luteal phase supplementation: a prospective randomized trial. Reproductive BioMedicine Online 2008;16(3):361‐7. - PubMed
-
- Dal Prato L, Borini A, Bonu MA, Maccolini A, Cattoli M, Flamigni C. Luteal support in IVF: i.m. versus intravaginal progesterone. Human Reproduction. 2004 Suppl 1; Vol. 19:i69‐70 (Abs #O‐198).
Doody 2009 {published data only}
-
- Doody KJ, Schnell VL, Foulk RA, Miller CE, Kolb BA, Blake EJ, et al. Endometrin for luteal phase support in a randomized, controlled, open‐label, prospective in‐vitro fertilization trial using a combination of Menopur and Bravelle for controlled ovarian hyperstimulation. Fertility and Sterility 2009;91(4):1012‐7. - PubMed
Drakakis 2007 {published data only}
-
- Drakakis P, Loutradis D, Vomvolaki E, Stefanidis K, Kiapekou E, Anagnostou E, et al. Luteal estrogen supplementation in stimulated cycles may improve the pregnancy rate in patients undergoing in vitro fertilization/intracytoplasmic sperm injection‐embryo transfer. Gynecological Endocrinology 2007;23(11):645‐52. - PubMed
Dunstone 1999 {published data only}
-
- Dunstone T, Zosmer A, Hussain S, Tozer A, Paney N, Wilson C, et al. A comparison between Cyclogest pessaries and Crinone gel as luteal support in IVF‐ET cycles [abstract]. British Fertility Society Annual Meeting Abstract Book. 1999:62 (Abs # FC21).
Elgindy 2010 {published data only}
-
- Elgindy EA, El‐Haieg DO. Does luteal estradiol supplementation have a role in long agonist cycles? Does luteal estradiol supplementation have a role in long agonist cycles? [abstract]. Fertility and Sterility. 2010; Vol. 88, issue Suppl 1:164 (Abs #168). - PubMed
-
- Elgindy EA, El‐Haieg DO, Mostafa MI, Shafiek M. Does luteal estradiol supplementation have a role in long agonist cycles?. Fertility and Sterility 2010;93(7):2182‐8. - PubMed
Engmann 2008 {published data only}
-
- Engmann L, DiLuigi A, Schmidt D, Benadiva C, Maier D, Nulsen J. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study. Fertility and Sterility 2008;89(3):554‐61. - PubMed
Erdem 2013 {published data only}
-
- Erdem M, Kutlusoy F, Erdem A, Guler I, Mesut O, Biberoglu K. Luteal phase support with estrogen in addition to progesterone in patients with poor response to gonadotropins undergoing IVF. Fertility and Sterility 2013;100(Suppl 1):S300‐1 (Abs # P‐526).
Fatemi 2006 {published data only}
-
- Fatemi HM, Kolibianakis EM, Camus M, Tournaye H, Donoso P, Papanikolaou E, et al. Addition of estradiol to progesterone for luteal supplementation in patients stimulated with GnRH antagonist/rFSH for IVF: a randomized controlled trial. Human Reproduction 2006;21(10):2628‐32. - PubMed
-
- Fatemi HM, Kolibianakis EM, Camus M, Tournaye H, Steirteghem A, Devroey P. Progesterone versus progesterone combined with estradiol as luteal support in cycles stimulated with GnRH antagonist/rec‐FSH for IVF: a randomized clinical trial [abstract]. Fertility and Sterility. 2005; Vol. 84, issue Suppl 1:s322 (Abs #P‐475).
Feichtinger 2011 {published data only}
-
- Feichtinger M, Hejek J, Kemter P, Feichtinger W. Effect of luteal phase support comparing early (day 1) and late (day 4) initiation with pregnancy rates. Journal of Reproductive Medicine and Endocrinology 2011;8(4):288‐90.
Friedler 1999 {published data only}
-
- Friedler S, Raziel A, Schachter M, Cohen O, Yaron M, Tartakovsky L, et al. Characteristics of conceptional and non‐conceptional cycles after IVF using micronized progesterone for luteal support: a comparative study of vaginal or oral administration [abstract]. Abstract Book 1. 1998; Vol. 13, issue Abstract Book 1:161 (Abs # P‐063).
-
- Friedler S, Raziel A, Schachter M, Strassburger D, Bukovsky I, Ron‐El R. Luteal support with micronized progesterone following in‐vitro fertilization using a down‐regulation protocol with gonadotrophin‐releasing hormone agonist: a comparative study between vaginal and oral administration. Human Reproduction 1999;14(8):1944‐8. - PubMed
Fujimoto 2002 {published data only}
-
- Fujimoto A, Osuga Y, Fujiwara T, Yano T, Tsutsumi O, Momoeda M, et al. Human chorionic gonadotropin combined with progesterone for luteal support improves pregnancy rate in patients with low late‐midluteal estradiol levels in IVF cycles. Journal of Assisted Reproduction and Genetics 2002;19(12):550‐4. - PMC - PubMed
-
- Fujimoto A, Osuga Y, Ooi N, Fujiwara T, Yano T, Taketani Y. Addition of hCG to progesterone as a luteal support improves pregnancy rates for patients with low mid‐luteal oestradiol levels in IVF and ICSI [abstract]. Human Reproduction. 2001; Vol. 16, issue Suppl 1:143 (Abs #P‐143).
Ganesh 2011 {published data only}
-
- Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogesterone with progesterone gel and micronized progesterone for luteal support in 1,373 women undergoing in vitro fertilization: a randomized clinical study. Fertility and Sterility 2011;95(6):1961‐5. [DOI: 10.1016/j.fertnstert.2011.01.148] - DOI - PubMed
Geber 2007 {published data only}
-
- Geber S, Maia L, Lauar I, Valle MP, Sampaio AC. Does recombinant LH combined to progesterone for luteal phase interfere in the outcome of assisted reproduction technique cycles? [abstract]. Fertility and Sterility 2007;88 Suppl 1:25 (Abs #66).
Geber 2007a {published data only}
-
- Geber S, Moreira ACF, Paula SOC, Sampaio M. Comparison of two different vaginal progesterone for luteal phase support in cycles of assisted reproduction. Jornal Brasileiro de Reproducao Assistida 2006;10(1):17‐21.
-
- Geber S, Moreira ACF, Paula SOC, Sampaio M. Comparison between two forms of vaginally administered progesterone for luteal phase support in assisted reproduction cycles. Reproductive BioMedicine Online 2007;14(2):155‐8. - PubMed
Geusa 2001 {published data only}
-
- Geusa S, Causio F, Marinaccio M, Stanziano A, Sarcina E. Luteal phase support with progesterone in IVF/ET cycles: a prospective, randomized study comparing vaginal and intramuscular administration [abstract]. Human Reproduction 2001;16 Suppl 1:145 (Abs # P‐111).
Golan 1993 {published data only}
-
- Golan A, Herman A, Soffer Y, Bukovsky I, Caspi E, Ron‐El R. Human chorionic gonadotrophin is a better luteal support than progesterone in ultrashort gonadotrophin‐releasing hormone agonist/menotrophin in‐vitro fertilization cycles. Human Reproduction 1993;8:1372‐5. - PubMed
Gorkemli 2004 {published data only}
-
- Gorkemli H, Ak D, Akyurek C, Aktan M, Duman S. Comparison of pregnancy outcomes of progesterone or progesterone plus estradiol for luteal phase support in ICSI‐ET cycles. Gynecologic and Obstetric Investigation 2004;58(3):140‐4. - PubMed
Goudge 2010 {published data only}
-
- Goudge CS, Nagel TC, Damario MA. Duration of progesterone‐in‐oil support after in vitro fertilization and embryo transfer: a randomized, controlled trial. Fertility and Sterility 2010;94(3):946‐51. - PubMed
Humaidan 2006 {published data only}
-
- Humaidan P, Bungum L, Bungum M, Andersen CY. Rescue of corpus luteum function with peri‐ovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: a pilot study. Reproductive BioMedicine Online 2006;13(2):173‐8. - PubMed
Hurd 1996 {published data only}
-
- Hurd WW, Randolph JF Jr, Christman GM, Ansbacher R, Menge AC, Gell JS. Luteal support with both estrogen and progesterone after clomiphene citrate stimulation for in vitro fertilization. Fertility and Sterility 1996;66:587‐92. - PubMed
Inamdar 2012 {published data only}
Isik 2009 {published data only}
-
- Isik AZ, Caglar GS, Sozen E, Akarsu C, Tuncay G, Ozbicer T, et al. Single‐dose GnRH agonist administration in the luteal phase of GnRH antagonist cycles: a prospective randomized study. Reproductive BioMedicine Online 2009;19(4):472‐7. - PubMed
Isikoglu 2007 {published data only}
-
- Isikoglu M, Ozgur K, Oehninger S. Extension of GnRH agonist through the luteal phase to improve the outcome of intracytoplasmic sperm injection. Journal of Reproductive Medicine 2007;52(7):639‐44. - PubMed
Iwase 2008 {published data only}
-
- Iwase A, Ando H, Toda S, Ishimatsu S, Harata T, Kurotsuchi S, et al. Oral progestogen versus intramuscular progesterone for luteal support after assisted reproductive technology treatment: a prospective randomized study. Archives of Gynecology and Obstetrics 2008;277(4):319‐24. - PubMed
Kably Ambe 2005 {published data only}
-
- Kably Ambe A, Ruiz Anguas J, Walters Arballo F, García Benitez CQ, Karchmer Krivitsky S. Results' analysis of estradiol and progesterone supplementation in luteal phase vs progesterone alone in an assisted reproduction program. Ginecologia y Obstetricia de Mexico 2005;73:173‐82. - PubMed
Kleinstein 2005 {published data only}
-
- Kleinstein J. Efficacy and tolerability of vaginal progesterone capsules (Utrogest 200) compared with progesterone gel (Crinone 8%) for luteal phase support during assisted reproduction. Fertility and Sterility 2005;83(6):1641‐9. - PubMed
-
- Kleinstein, J. Efficacy of Utrogest 200 and Crinone 8% for luteal phase support during ART: a comparative, multicenter study [Abstract]. Human Reproduction. 2004; Vol. 19:i123 (Abs #P‐357).
Kohls 2012 {published data only}
-
- Kohls G, Ruiz F, Martinez M, Hauzman E, Fuente G, Pellicer A, Garcia‐Velasco JA. Early progesterone cessation after in vitro fertilization/intracytoplasmic sperm injection: a randomized, controlled trial. Fertility and Sterility 2012;98:858‐62. - PubMed
-
- Kohls G, Ruiz FJ, Fuente G, Toribio M, Martinez M, Pellicer A, et al. Early progesterone cessation after in vitro fertilization. Human Reproduction 2010;25 Suppl 1(6):i249 Abstract no. P‐344.
Kupferminc 1990 {published data only}
-
- Kupferminc MJ, Lessing JB, Amit A, Yovel I, David MP, Peyser MR. A prospective randomized trial of human chorionic gonadotrophin or dydrogesterone support following in‐vitro fertilization and embryo transfer. Human Reproduction 1990;5(3):271‐3. - PubMed
Kyrou 2011 {published data only}
-
- Kyrou D, Fatemi HM, Zepiridis L, Riva A, Papanikolaou EG, Tarlatzis BC, Devroey P. Does cessation of progesterone supplementation during early pregnancy in patients treated with recFSH/GnRH antagonist affect ongoing pregnancy rates? A randomized controlled trial. Human Reproduction 2011;26(5):1020‐4. - PubMed
Lam 2008 {published data only}
-
- Lam PM, Cheung MC, Cheung LP, Lok HI, Haines CJ. Effects of early luteal‐phase vaginal progesterone supplementation on the outcome of in vitro fertilization and embryo transfer. Gynecological Endocrinology 2008;24(12):674‐80. - PubMed
Lewin 1994 {published data only}
-
- Lewin A, Benshushan A, Mezker E, Yanai N, Schenker JG, Goshen R. The role of estrogen support during the luteal phase of in vitro fertilization‐embryo transplant cycles: a comparative study between progesterone alone and estrogen and progesterone support. Fertility and Sterility 1994;62:121‐5. - PubMed
-
- Lewin A, Pisov G, Turgeman R, Fatum M, Shufaro Y, Simon A, et al. Simplified artificial endometrial preparation, using oral estradiol and novel vaginal progesterone tablets: a prospective randomized study. Gynecological Endocrinology 2002;16(2):131‐6. - PubMed
Licciardi 1999 {published data only}
-
- Licciardi F, Kwiatkowski A, Noyes N, Berkeley AS, Krey LL, Grifo JA. Oral versus intramuscular progesterone for in vitro fertilization: a prospective randomized study. Fertility and Sterility 1999;71:614‐8. - PubMed
Lin 2013 {published data only}
-
- Lin H, Li Y, Li L, Wang W, Zhang Q, Chen X, Yang D. Oral oestradiol supplementation as luteal support in IVF/ICSI cycles: a prospective, randomized controlled study. European Journal of Obstetrics Gynecology and Reproductive Biology 2013;167:171‐5. - PubMed
Lockwood 2014 {published data only}
-
- Lockwood G, Griesinger G, Cometti B. Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study. Fertility and Sterility 2014;101:112‐9. - PubMed
Loh 1996 {published data only}
-
- Loh SKE, Leong NKY. Luteal phase support in IVF‐cycles ‐ is intramuscular progesterone the therapy of choice? [abstract]. Fertility Society of Australia XV Annual Meeting Abstract Book (Abs #O24). 1996.
Ludwig 2001 {published data only}
-
- Ludwig M, Finas A, Bals‐Pratsch M, Felberbaum RE, Schopper B, Al‐Hasani S, et al. Prospective, randomized study to evaluate the pregnancy rate using HCG, vaginal progesterone (Utrogest), or a combination of both for luteal‐phase support: preliminary results [abstract]. Human Reproduction. 1999; Vol. 14 Suppl 1:2‐3 (Abs # O‐004).
-
- Ludwig M, Finas A, Katalinic A, Strik D, Kowalcek I, Schwartz P, et al. Prospective, randomized study to evaluate the success rates using hCG, vaginal progesterone or a combination of both for luteal phase support. Acta Obstetricia et Gynecologica Scandinavica 2001;80:574‐82. - PubMed
Ludwig 2002 {published data only}
-
- Ludwig M, Schwartz P, Babahan B, Katalinic A, Bals‐Pratsch M, Diedrich K. Progesterone gel (Crinone 8%) is more comfortable than progesterone suppositories (Utrogest) for luteal phase support and results in comparable pregnancy rates: results of a prospective, randomized study [abstract]. Fertility and Sterility. 2000; Vol. 74:S210 (Abs #P‐S210).
-
- Ludwig M, Schwartz P, Babahan B, Katalinic A, Weiss JM, Felberbaum R, et al. Luteal phase support using either Crinone 8% or Utrogest: results of a prospective, randomized study. European Journal of Obstetrics and Gynaecology 2002;103:48‐52. - PubMed
-
- Schwartz P, Ludwig M, Babahan B, Katalinic A, Bals‐Pratsch M, Felberbaum R, et al. Luteal phase support using either progesterone gel (Crinone 8%) or progesterone suppositories (Utrogest): results of a prospective, randomized study [abstract]. Human Reproduction 2000;15(Abstract Book 1):43‐4 (Abs #O‐111).
Macrolin 1993 {published data only}
-
- Macrolin G, Buvat J, Guittard C, Herbaut JC, Louvet AL, Dehaene JL. [Fécondation in vitro après agoniste de la LHRH: comparison randomisée de soutiens lutéaux par progestérone vaginale seule ou associee a la gonadotrophine chorionique]. Contraception Fertilité Sexualité 1993;21(5):434.
Martinez 2000 {published data only}
-
- Martinez F, Coroleu B, Parera N, Alvarez M, Traver JM, Boada M, et al. Human chorionic gonadotropin and intravaginal natural progesterone are equally effective for luteal phase support in IVF. Gynaecological Endocrinology 2000;14:316‐20. - PubMed
Miller 2010 {published data only}
-
- Miller CE, Doody KJ, Zbella E, Webster B, Bush M, Scobey J. Efficacy of vaginal progesterone inserts (Endometrin) compared to intramuscular progesterone in oil for luteal support in IVF patients. Fertility and Sterility 2010;94 Suppl 1(4):20‐1 Abstract no. O‐68.
Mochtar 2006 {published data only}
-
- Mochtar MH, Wely M, Veen F. Timing luteal phase support in GnRH agonist down‐regulated IVF/embryo transfer cycles. Human Reproduction 2006;21(4):905‐8. - PubMed
Moini 2011 {published data only}
Nallapeta 2013 {published data only}
-
- Nallapeta S, Sharma V. Intra‐muscular progesterone as a luteal phase support increases live birth rate as compared to vaginal route. Fertility and Sterility 2013;100(Supp):S8 (Abs #O‐25).
-
- Nallapeta S, Sharma V. Relationship of different progesterone preparations on ovarian volumes in women undergoing IVF/ICSI treatment. Fertility and Sterility 2013;100(Suppl):S60 (Abs # O‐196).
Ng 2003 {published data only}
-
- Ng EHY, Miao B, Cheung W, Ho PC. A randomised comparison of side effects and patient inconvenience of two vaginal progesterone formulations used for luteal support in in vitro fertilisation cycles. European Journal of Obstetrics Gynecology and Reproductive Biology 2003;111:50‐4. - PubMed
Ng 2007 {published data only}
-
- Ng EHY, Chan CCW, Tang OS, Ho PC. A randomized comparison of side effects and patient convenience between Cyclogest suppositories and Endometrin tablets used for luteal phase support in IVF treatment. European Journal of Obstetrics Gynecology and Reproductive Biology 2007;131(2):182‐8. - PubMed
Nyboe Andersen 2002 {published data only}
-
- Nyboe Andersen A, Popovic‐Todorovic B, Schmidt KT, Loft A, Lindhard A, Hojgaard A, et al. Progesterone supplementation during early gestations after IVF or ICSI has no effect on the delivery rates: a randomized controlled trial. Human Reproduction 2002;17:357‐61. - PubMed
Patki 2007 {published data only}
-
- Patki A, Pawar VC. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecological Endocrinology 2007;23 Suppl 1:68‐72. - PubMed
Perino 1997 {published data only}
-
- Perino M, Brigandi A, Abate FG, Costabile L, Balzano E, Abate A. Intramuscular versus vaginal progesterone in assisted reproduction: a comparative study. Clinical and Experimental Obstetrics and Gynecology 1997;24:228‐31. - PubMed
Porcu 2003 {published data only}
-
- Porcu E. Intramuscular versus vaginal progesterone in assisted reproduction [abstract]. Fertility and Sterility. 2003; Vol. 80:S131 (Abs # P‐32).
Pouly 1996 {published data only}
-
- Pouly JL, Bassil S, Frydman R, Hedon B, Nicollet B, Prada Y, et al. Luteal phase support after vaginal progesterone: comparative study with micronized oral progesterone. Contraception, Fertilité, Sexualité 1997;25:596‐601. - PubMed
-
- Pouly JL, Bassil S, Frydman R, Hedon B, Nicollet B, Prada Y, et al. Luteal support after in‐vitro fertilization: Crinone 8%, a sustained release vaginal progesterone gel, versus Utrogestan, an oral micronized progesterone. Human Reproduction 1996;11:2085‐9. - PubMed
Propst 2001 {published data only}
-
- Propst AM, Hill JA, Ginsburg ES, Hurwitz S, Politch J, Yanushpolsky EH. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2001;76:1144‐9. - PubMed
-
- Propst AM, Hill JA, Politch J, Yanushpolsky EH. A prospective, randomized study comparing Crinone and intramuscular progesterone supplementation in IVF/ET cycles [abstract]. Fertility and Sterility. 2000; Vol. 74:s30‐1 (Abs #O‐084). - PubMed
Qublan 2008 {published data only}
-
- Qublan H, Amarin Z, Al‐Quda M, Diab F, Nawasreh M, Malkawi S, et al. Luteal phase support with GnRH‐a improves implantation and pregnancy rates in IVF cycles with endometrium of [less‐than or equal to]7 mm on day of egg retrieval. Human Fertility 2008;11(1):43‐7. - PubMed
Rodriguez‐Pezino 2004 {published data only}
-
- Rodriguez‐Pezino J, Saucedo‐de la Llata E, Batiza‐Resendiz V, Galache‐Vega P, Santos‐Haliscak R, Hernandez‐Ayup S, et al. Vaginal progesterone in assisted reproduction. Human Reproduction. Berlin, Germany, 2004; Vol. 19 Suppl 1:i51.
Salehpour 2013 {published data only}
Saucedo 2000 {published data only}
-
- Saucedo‐de la Llata E, Galache VP, Hernandez AS, Santos HR, Arenas ML, Patrizio P. Randomized trial of three different forms of progesterone supplementation in ART: preliminary results [abstract]. Fertility and Sterility 2000;74 Suppl 1:S150 (Abs # P‐175).
Saucedo 2003 {published data only}
-
- Saucedo‐de la Llata E, Batiza V, Arenas L, Santos R, Galache P, Hernandez‐Ayup S, et al. Progesterone for luteal support: randomized, prospective trial comparing vaginal and i.m. administration [abstract]. Fertility and Sterility 2003;18 Suppl 1:130 (Abs # P‐382).
Serna 2008 {published data only}
-
- Serna J, Cholquevilque JL, Cela V, Martinez‐Salazar J, Requena A, Garcia‐Velasco JA. Estradiol supplementation during the luteal phase of IVF‐ICSI patients: a randomized, controlled trial. Fertility and Sterility 2008;90(6):2190‐5. - PubMed
Serour 2012 {published data only}
-
- Serour AG. Luteal phase support in fresh IVF/ICSI cycles. International Journal of Gynecology and Obstetrics 2012;119:S533 (Abs# M007).
Stadtmauer 2013 {published data only}
-
- Howard B, Weiss H, Doody K. Efficacy of a progesterone vaginal ring compared to a vaginal gel for luteal phase supplementation in patients with and without risk factors for poor ovarian response. Human Reproduction 2012;27:Abs# P‐338.
-
- Perloe M, Weiss H, Howard B. Impact of luteal supplementation with a weekly progesterone vaginal ring during in vitro fertilization (IVF) by day of embryo transfer (ET). Fertility and Sterility 2012;98(S1):S4‐5 (Abs # O‐14).
-
- Schnell V, Howard B, Weiss H. Number of embryos transferred and multiple pregnancy rates in a randomized study of progesterone vaginal ring versus gel for luteal support following in vitro fertilization. Fertility and Sterility 2013;99(3):S36‐S37. - PubMed
-
- Silverberg KM, Reape KZ, Howard BK. Efficacy of a progesterone vaginal ring versus progesterone gel for luteal phases supplementation by body mass index (BMI). Fertility and Sterility 2011;96 Suppl:S279 (Abs# P‐538).
-
- Stadtmauer L, Silverberg KM, Ginsburg ES, Weiss H, Howard B. Progesterone vaginal ring versus vaginal gel for luteal support with in vitro fertilization: a randomized comparative study. Fertility and Sterility 2013;99:1543‐9. - PubMed
Strehler 1999 {published data only}
-
- Strehler E, Abt M, El‐Danasouri I, Sterzik K. Transvaginal administration of micronized progesterone does not differ to progesterone gel application in the efficacy of luteal phase support in IVF cycles [abstract]. 11th World Congress of In Vitro Fertilization and Human Reproductive Genetics Abstract Book. 1999:287 (Abs # P‐243).
Sumita 2003 {published data only}
-
- Sumita S, Sofat S Sr. Intramuscular versus intra vaginal progesterone as luteal phase and early pregnancy support in patients undergoing IVF‐ET [abstract]. Fertility and Sterility 2003;80 Suppl 3:134‐5 (Abs # P‐44).
Tay 2005 {published data only}
-
- Tay PYS, Lenton EA. The impact of luteal supplement on pregnancy outcome following stimulated IVF cycles. Medical Journal of Malaysia 2005;60(2):151‐7. - PubMed
Tesarik 2006 {published data only}
-
- Tesarik J, Hazout A, Mendoza‐Tesarik R, Mendoza N, Mendoza C. Beneficial effect of luteal‐phase GnRH agonist administration on embryo implantation after ICSI in both GnRH agonist‐ and antagonist‐treated ovarian stimulation cycles. Human Reproduction 2006;21(10):2572‐9. - PubMed
Tonguc 2011 {published data only}
-
- Esra T, Var T, Citil A, Dogan M, Cicek N. Estradiol supplementation in luteal phase: how much matter?. Human Reproduction. Rome, Italy, 2010; Vol. 25 Suppl 1:i307‐8.
-
- Tonguc E, Var T, Ozyer S, Citil A, Dogan M. Estradiol supplementation during the luteal phase of in vitro fertilization cycles: a prospective randomised study. European Journal of Obstetrics Gynecology and Reproductive Biology 2011;154:172‐6. - PubMed
Torode 1987 {published data only}
-
- Torode HW, Porter RN, Vaughan JI, Saunders DM. Luteal phase support after in vitro fertilisation: a trial and rationale for selective use. Clinical Reproduction and Fertility 1987;5:255‐61.
Ugur 2001 {published data only}
-
- Ugur M, Yenicesu O, Ozcan S, Keles G, Gokmen O. A prospective randomized study comparing hCG, vaginal micronized progesterone and a combination regimen for luteal phase support in an in‐vitro fertilization programme [abstract]. Fertility and Sterility 2001;76 Suppl 1:118 (Abs # P‐19).
Vimpeli 2001 {published data only}
-
- Vimpeli T, Tinkanen H, Huhtala H, Ronnberg L, Kujansuu E. Salivary and serum progesterone concentrations during two luteal support regimens used in in vitro fertilization treatment. Fertility and Sterility 2001;76:847‐8. - PubMed
Williams 2001 {published data only}
-
- Williams SC, Oehninger S, Gibbons WE, Cleave WC, Muasher SJ. Delaying the initiation of progesterone supplementation results in decreased pregnancy rates after in vitro fertilization: a randomized, prospective study. Fertility and Sterility 2001;76:1140‐3. - PubMed
Wong 1990 {published data only}
-
- Wong YF, Loong EPL, Mao KR, Tam PPL, Panesar NS, Neale E, et al. Salivary oestradiol and progesterone after in vitro fertilization and embryo transfer using different luteal support regimens. Reproduction Fertility and Development 1990;2:351‐8. - PubMed
Yanushpolsky 2010 {published data only}
-
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Compared to Crinone, intramuscular progesterone (IMP) delays menstrual bleeding but does not improve pregnancy rates or outcomes in IVF/ET cycles. Fertility and Sterility. 2009; Vol. 92 Suppl 1:243.
-
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization‐embryo transfer cycles: a prospective randomized study. Fertility and Sterility 2010;94(7):2596‐9. - PubMed
-
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Patterns of luteal phase bleeding in in vitro fertilization cycles supplemented with Crinone vaginal gel and with intramuscular progesterone ‐ Impact of luteal estrogen: prospective, randomized study and post hoc analysis. Fertility and Sterility 2011;95(2):617‐20. - PubMed
-
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein MD. Comparison of Crinone 8% intravaginal gel and intramuscular progesterone supplementation for in vitro fertilization/embryo transfer in women under age 40: interim analysis of a prospective randomized trial. Fertility and Sterility 2008;89(2):485‐7. - PubMed
Yildiz 2014 {published data only}
-
- Yildiz, Gulsah Aynaoglu, Sukur, Yavuz Emre, Ates, Can, Aytac, Rusen. The addition of gonadotrophin releasing hormone agonist to routine luteal phase support in intracytoplasmic sperm injection and embryo transfer cycles: a randomized clinical trial.. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2014;182:66‐70. - PubMed
Zegers‐Hochschild 2000 {published data only}
-
- Zegers‐Hochschild F, Balmaceda JP, Fabres C, Alam V, Mackenna A, Fernandez E, et al. Efficacy and acceptability of a vaginal ring releasing progesterone for in‐vitro fertilization and oocyte donation [abstract]. Human Reproduction 1998;13(Abstract Book 1):118‐9 (Abs #O‐231). - PubMed
-
- Zegers‐Hochschild F, Balmaceda JP, Fabres C, Alam V, Mackenna A, Fernández E, et al. Prospective randomized trial to evaluate the efficacy of a vaginal ring releasing progesterone for IVF and oocyte donation. Human Reproduction 2000;15(10):2093‐7. - PubMed
References to studies excluded from this review
Abu‐Musa 2008 {published data only}
-
- Abu‐Musa A, Usta I, Nassar A, Hajami F, Hannoun A. Effect of 17alpha‐hydroxyprogesterone caproate before embryo transfer on the outcome of in vitro fertilization and embryo transfer: a randomized trial. Fertility and Sterility 2008;89(5):1098‐102. - PubMed
Abu‐Musa 2008a {published data only}
-
- Abu‐Musa A, Usta I, Nassar A, Hajami F, Hannoun A. Effect of 17alpha‐hydroxyprogesterone caproate before embryo transfer on the outcome of in vitro fertilization and embryo transfer: a randomized trial. Fertility and Sterility 2008;89(5):1098‐102. - PubMed
Aleyasin 2012 {published data only}
-
- Aleyasin A, Mahdavi A, Agha Hosseini M, Safdarian L, Fallahi P, Bahmaee F. Comparison of two doses of recombinant human chorionic gonadotropin and urinary human chorionic gonadotropin during intracytoplasmic sperm injection cycles. Human Reproduction 2012;27:P‐324.
Allahbadia 2004 {published data only}
-
- Allahbadia GN, Kaur K, Kadam K, Virk S, Gandhi G, Gosrani S. The comparison of pregnancy outcomes of intramuscular progesterone versus oral dydrogesterone for luteal phase support in donor egg IVF recipient cycles. Fertility and Sterility 2004;82 Suppl 2:194.
Allen 2004 {published data only}
-
- Allen C, Harrison RF. Luteal support progesterone vaginal gel v pessary: clinical/endocrine outcome. Human Reproduction 2004;19 Suppl:i125‐6.
Alsanie 2005 {published data only}
-
- Alsanie A, Kadoch I, Phillips S, Lapensee L, Hemmings R, Bissonnette F. Adding estrogen to progesterone in luteal phase support in vitro fertilization‐embryo transfer (IVF‐ET) cycles produces pregnancies with higher quantitative beta human chorionic gonadotropins (beta hCG). Fertility and Sterility 2005;84 Suppl:155.
Andersen 2014 {published data only}
-
- Andersen CY, Andersen KV. Improving the luteal phase after ovarian stimulation: reviewing new options. Reproductive Biomedicine Online 2014;28(5):552‐9. - PubMed
Anserini 2001 {published data only}
-
- Anserini P, Costa M, Remorgida V, Sarli R, Guglielminetti E, Ragni N. Luteal phase support in assisted reproductive cycles using either vaginal (Crinone 8) or systemic (Prontogest) progesterone: results of a prospective randomized study. Minerva Ginecologica 2001;53:297‐301. - PubMed
Anthony 1993 {published data only}
-
- Anthony FW, Smith EM, Gadd SC, Masson GM, Chard T, Perry L. Placental protein 14 secretion during in vitro fertilization cycles with and without human chorionic gonadotropin for luteal support. Fertility and Sterility 1993;59:187‐91. - PubMed
Araujo 1994 {published data only}
-
- Araujo E Jr, Bernardini L, Frederick JL, Asch RH, Balmaceda JP. Prospective randomized comparison of human chorionic gonadotropin versus intramuscular progesterone for luteal‐phase support in assisted reproduction. Journal of Assisted Reproduction and Genetics 1994;11(2):74‐8. - PubMed
Araujo Filho 1996 {published data only}
-
- Araujo Filho E, Asch RH, Araujo E, Luz OA, Balmaceda JP. Prospective and randomized trial comparing human chorionic gonadotropin and intramuscular progesterone for luteal phase support in assisted fertilization [Estudo prospectivo e randomizado comparando gonadotrofina coriônica humana e progesterona intramuscular para suporte da fase lútea em reproduçao assistida]. Revista Brasileira de Ginecologia e Obstetrica 1996;18(2):131‐7.
Baber 1988 {published data only}
-
- Baber RJ, Kuan R, Porter RN, Saunders DM. Early pregnancy support in an in vitro fertilization program: does human chorionic gonadotropin reduce the miscarriage rate?. Asia‐Oceania Journal of Obstetrics and Gynaecology 1988;14:453‐5. - PubMed
Beckers 2006 {published data only}
-
- Beckers NGM, Platteau P, Eijkemans MJ, Macklon NS, Jong FH, Devroey P, et al. The early luteal phase administration of estrogen and progesterone does not induce premature luteolysis in normo‐ovulatory women. European Journal of Endocrinology 2006;155(2):355‐63. - PubMed
Belaisch‐Allart 1988 {published data only}
-
- Belaisch‐Allart J, Mouzon J. Effect of luteal phase supplementation in an IVF programme after ovarian stimulation by LH‐RH analogs. Multicentric analysis [Effet de la supplementation de la phase luteale dans un programme de fecondation in vitro apres stimulation de l'ovulation par les agonistes du LHRH. Etude multicentrique]. Contraception, Fertilite, Sexualite 1988;16(7):654‐6.
Ben‐Nun 1990 {published data only}
-
- Ben‐Nun I, Ghetler Y, Jaffe R, Siegal A, Kaneti H, Fejgin M. Effect of preovulatory progesterone administration on the endometrial maturation and implantation rate after in vitro fertilization and embryo transfer. Fertility and Sterility 1990;53:276‐81. - PubMed
Berjis 2008 {published data only}
-
- Berjis K, Sarem A, Moaya M, Mohamad Alayha N. The comparative assessment of intramuscular progesterone and intravaginal progesterone to support luteal phase in IVF cycle [Farsi]. Medical Sciences Journal of the Islamic Azad University of Tehran Medical Unit 2008;18(1):9.
Bjuresten 2011 {published data only}
-
- Bjuresten K, Landgren BM, Hovatta O, Stavreus‐Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertility and Sterility 2011;95:534‐7. - PubMed
Blake 2010 {published data only}
-
- Blake EJ, Norris PM, Dorfman SF, Longstreth J, Yankov VI. Single and multidose pharmacokinetic study of a vaginal micronized progesterone insert (Endometrin) compared with vaginal gel in healthy reproductive‐aged female subjects. Fertility and Sterility 2010;94(4):1296‐301. - PubMed
Buvat 1988 {published data only}
-
- Buvat J, Marcolin G, Herbaut JC, Dehaene JL, Verbecq P, Fourlinnie JC. A randomized trial of human chorionic gonadotropin support following in vitro fertilization and embryo transfer. Fertility and Sterility 1988;49:458‐61. - PubMed
-
- Macrolin G, Buvat J, Herbaut JC, Louvet AL, Dehaene JL, Renouard O. Luteal phase support with HCG ‐ can it be of any benefit following in vitro fecundation (IVF)? A controlled randomized study covering 116 cycles [Le soutien de la phase lutéale par HCG a‐t‐il de l'intérêt après fécondation in vitro?]. Gynecologie 1988;39:163‐6.
Buvat 1990 {published data only}
-
- Buvat J, Marcolin G, Guittard C, Dehaene JL, Herbaut JC, Louvet AL. Luteal support after administration of an LHRH analog for in vitro fertilization. Superiority of vaginal progesterone in comparison with oral progesterone [Soutien lutéal après analogue de la gonadoréline pour fécondation in vitro. Supériorité de la progestérone vaginale sur la progestérone orale]. La Presse Médicale 1990;19:527. - PubMed
-
- Buvat J, Marcolin G, Guittard C, Herbaut JC, Louvet AL, Dehaene JL. Luteal phase support after LHRH‐agonist for in vitro fertilization (IVF): vaginal progesterone is superior to oral progesterone and as much effective as human chorionic gonadotropin (hCG) [Soutien lutéal après LHRH‐agonistes pour fécondation in vitro: la progestérone vaginale est supérieure à la progestérone orale, et aussi efficace que la gonadotrophine chorionique (hCG)]. Contraception Fertilite Sexualite 1990;18:616‐7.
-
- Buvat J, Marcolin G, Guittard C, Herbaut JC, Louvet AL, Dehaene JL. Luteal support after luteinizing hormone‐releasing hormone agonist for in vitro fertilization: superiority of human chorionic gonadotropin over oral progesterone. Fertility and Sterility 1990;53:490‐4. - PubMed
-
- Buvat J, Mercolin G, Guittard C, Dehaene JL, Verbecq P, Renouard O, et al. Chorionic gonadotropin support of the luteal phase following in vitro fertilization and embryo transfer. Randomized comparison wih oral progesteronein protocols using triptoreline [Soutien de la phase lutéal par la gonadotrophine chorionique après fécondation in vitro et transfert d'embryon. Comparaison randomisée à la progestérone per os dans les protocoles utilitsant la triptoréline]. La Presse Médicinale 1989;18:539. - PubMed
Casini 2003 {published data only}
-
- Casini ML, Unfer V, Costabile L, Gerli S, Agostini R, Renzo GC. Oral versus i.m. progesterone supplementation in IVF‐embryo transfer cycles: a randomized study [abstract]. Human Reproduction 2003;18 Suppl 1:106 (Abs # P‐307).
Chakravarty 2012 {published and unpublished data}
-
- Chakravarty A, Sharma Palchaudhuri S, Chakraborty P, Goswami SK, Chattopadhyay R, Chakravarty B. Role of estrogen as luteal phase support (LPS) in normal and expected poor responders in long agonist in vitro fertilization (IVF)/inta‐cytoplasmic sperm injection (ICSI) cycles. Fertility and Sterility 2012;98(Suppl 1):S257 (Abs # P‐491).
Chang 2008 {published data only}
-
- Chang S‐P. Comparison of Crinone 8% intravaginal gel and intramuscular progesterone for luteal support in in vitro fertilization. Journal of the Chinese Medical Association 2008;71(8):381‐5. - PubMed
Chang 2009 {published data only}
Chantilis 1999 {published data only}
-
- Chantilis SJ, Zeitoun KM, Patel SI, Johns DA, Madziar VA, McIntire DD. Use of Crinone vaginal progesterone gel for luteal support in in vitro fertilization cycles. Fertility and Sterility 1999;72:823‐9. - PubMed
Check 2010 {published data only}
-
- Check J H, Dietterich C, Cohen R, Choe J K, Amui J, Brasile D. Increasing the dosage of progesterone (P) supplemention from the mid‐luteal phase in women not attaining a mid‐luteal homogeneous hyperechogenic (HH) pattern with sonography improves pregnancy rates (PRS) following frozen embryo transfer (ET). Clinical and Experimental Obstetrics and Gynecology 2010;37(1):13‐4. - PubMed
Check 2012 {published data only}
-
- Check JH. Luteal phase support for in vitro fertilization‐embryo transfer ‐ present and future methods to improve successful implantation. Clinical and Experimental Obstetrics & Gynecology 2012;39(4):422‐8. - PubMed
Check 2013 {published data only}
-
- Check JH, Wilson C, Levine K, Cohen R, Corley D. Improved implantation and live delivered pregnancy rates following transfer of embryos derived from donor oocytes by single injection of leuprolide in mid‐luteal phase. Fertility and Sterility 2013;100(3 Suppl):S301. - PubMed
Claman 1992 {published data only}
-
- Claman P, Domingo M, Leader A. Luteal phase support in in‐vitro fertilization using gonadotrophin releasing hormone analogue before ovarian stimulation: a prospective randomized study of human chorionic gonadotrophin versus intramuscular progesterone. Human Reproduction 1992;7(4):487‐9. - PubMed
-
- Claman P, Domingo M, Leader A, Gauthier C. Preliminary results suggest an advantage to using human chorionic gondotropin over progesterone treatment to support the luteal phase after treatment with leuprolide acetate and human menopausal gonadotrophin for superovulation in IVF‐ET. Fertility & Sterility 1991;54(116).
Costabile 2001 {published data only (unpublished sought but not used)}
-
- Costabile L, Gerli S, Manna C, Rossetti D, Renzo GC, Unfer V. A prospective randomized study comparing intramuscular progesterone and 17alpha‐hydroxyprogesteronecaproate in patients undergoing in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2001;76(2):964‐6. - PubMed
Daya 2009 {published data only}
-
- Daya S. Luteal support: progestogens for pregnancy protection. Maturitas 2009;65 Suppl 1:29‐34. - PubMed
Demir 2013 {published data only}
-
- Demir B, Dilbaz S, Cinar O, Ozdegirmenci O, Dede S, Dundar B, Goktolga U. Estradiol supplementation in intracytoplasmic sperm injection cycles with thin endometrium. Gynecological Endocrinology 2013;29(1):42‐5. - PubMed
Demirel 2003 {published data only}
-
- Demirel LC, Baltaci V, Aydos K, Aytaç R, Satiroglu H, Ünlü C. A randomized comparison of the timing for initiation of luteal phase support. Human Reproduction 2003;18(Suppl 1):130 (Abs # P‐382).
Ding 2005 {published data only}
-
- Ding J, Rana N, Dmowski W. Comparative effectiveness of two luteal phase support protocols for IVF. Fertility and Sterility 2005;84 Suppl 1:349.
Ellenbogen 2011 {published data only}
-
- Ellenbogen A, Atamny R, Fainaru O, Meidan E, Rotfarb N, Michaeli M. In vitro maturation of oocytes: a novel method of treatment of patients with polycystic ovarian syndrome undergoing in vitro fertilization. Harefuah 2011;150(11):833‐76. - PubMed
Erman Akar 2005 {published data only}
-
- Erman Akar M, Kursun S, Taskin O, Simsek M, Kaba M, Uner M. Intravaginal progesterone gel vs 17oe hydroxyprogesterone caproate in ICSI embryo transfer cycles: a prospective randomized study. Fertility and Sterility 2005;84 Suppl 1:320.
Escriba 2006 {published data only}
-
- Escriba MJ, Bellver J, Bosch E, Sanchez M, Pellicer A, Remohi J. Delaying the initiation of progesterone supplementation until the day of fertilization does not compromise cycle outcome in patients receiving donated oocytes: a randomized study. Fertility and Sterility 2006;86(1):92‐7. - PubMed
Farhi 2000 {published data only}
-
- Farhi J, Nahum H, Steinfeld Z, Shorer M, Zakut H. Do adequate E2 and progesterone levels during luteal phase promote or merely reflect implantation in IVF cycles. Fertility & Sterility 1998;70(3):Abs # S‐429.
-
- Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2000;73:761‐6. - PubMed
Farrag 2008 {published data only}
FeiYang 2013 {published data only (unpublished sought but not used)}
-
- Fei Yang D, Jia Yin L. Different LH add‐back and luteal phase supplement influence clinical outcome in GnRH antagonist protocol ‐ a prospective RCT study in fresh and frozen transfer cycles. Fertility and Sterility 2013;100(Suppl):S270 (Abs # P‐424).
Feliciani 2004 {published data only}
-
- Feliciani E, Ferraretti AP, Balicchia B, Grieco N, Magli MC, Gianaroli A. A prospective randomised study comparing the effect of intravaginal progesterone and intramuscular progesterone in frozen/thawed embryo transfer (FET) cycles. Human Reproduction 2004;82:i51.
Gallardo 2004 {published data only}
-
- Gallardo LE, Ayón P, Neuspiller F. [Estudio de dos vías diferentes de administración de progesterona micronizada en reproducción asistida]. Ginecología y Obstetricia de México 2004;72(8):407‐10. - PubMed
Garcia‐Velasco 2009 {published data only}
-
- Garcia‐Velasco J, Motta L, Lopez A, Mayoral M, Cerrillo M, Pellicer A, Pacheco A. Estradiol/progesterone vs low dose hCG luteal phase support in GnRH agonist triggered ART cycles: a pilot study. Human Reproduction 2010;25 Suppl 1:i86 (Abs #O‐224).
-
- Garcia‐Velasco JA, Motta L, Lopez A, Mayoral M, Cerrillo M, Pacheco A. Low‐dose human chorionic gonadotropin versus estradiol/progesterone luteal phase support in gonadotropin‐releasing hormone agonist‐triggered assisted reproductive technique cycles: understanding a new approach. Fertility and Sterility 2010;94:2820‐3. - PubMed
-
- Garcia‐Velasco JA, Quea G, Piro M, Mayoral M, Ruiz M, Toribio M, et al. Letrozole administration during the luteal phase after ovarian stimulation impacts corpus luteum function: a randomized, placebo‐controlled trial. Fertility and Sterility 2009;92(1):222‐5. - PubMed
Gazvani 2012 {published data only}
Germond 2002 {published data only}
-
- Germond M, Capelli P, Bruno G, Vesnaver S, Senn A, Rouge N, Biollaz J. Comparison of the efficacy and safety of two formulations of micronized progesterone (Ellios and Utrogestan) used as luteal phase support after in vitro fertilization. Fertility and Sterility 2002;77(2):313‐7. - PubMed
Ghanem 2009 {published data only}
-
- Ghanem M, Sadek E, Helal A, Gamal A, Eldiasty E, Bakre NI, Houssen M. The effect of luteal phase support protocol on luteal phase serum estradiol and progesterone and cycle outcome in ICSI cycles: a randomized trial [Abstract]. Human Reproduction. 2008; Vol. 23, issue Suppl 1:i124 (Abs #P‐307).
-
- Ghanem ME, Sadek EE, Elboghdady LA, Helal AS, Gamal A, Eldiasty A, et al. The effect of luteal phase support protocol on cycle outcome and luteal phase hormone profile in long agonist protocol intracytoplasmic sperm injection cycles: a randomized clinical trial. Fertility and Sterility 2009;92(2):486‐93. - PubMed
Gibbons 1998 {published data only}
-
- Gibbons W E, Toner J P, Hamacher P, Kolm P. Experience with a novel vaginal progesterone preparation in a donor oocyte program. Fertility and Sterility 1998;69:96‐101. - PubMed
Griesinger 2006 {published data only}
-
- Griesinger G, Diedrich K. Vaginal progesterone for luteal phase support in assisted reproduction [Die vaginale Anwendung von natürlichem Progesteron als Lutealphasenunterstützung nach IVF und Embryotransfer]. Geburtsh Frauenheilk 2006;66:655‐64.
Herman 1990 {published data only}
-
- Herman A, Ron‐El R, Golan A, Raziel A, Soffer Y, Caspi E. Pregnancy rate and ovarian hyperstimulation after luteal human chorionic gonadotropin in in vitro fertilization stimulated with gonadotropin‐releasing hormone analog and menotropins. Fertility and Sterility 1990;53:92‐6. - PubMed
Herman 1996 {published data only}
-
- Herman A, Raziel A, Nachum H, Strassburger D, Soffer Y, Bukovsky Y, et al. The benefits of midluteal addition of human chorionic gonadotrophin in IVF using a down‐regulation protocol and luteal support with progesterone [abstract]. Human Reproduction 1995;10(Abstract Book 2):63 (Abs #127).
-
- Herman A, Raziel A, Strassburger D, Soffer Y, Bukovsky I, Ron‐El R. The benefits of mid‐luteal addition of human chorionic gonadotrophin in in‐vitro fertilization using a down‐regulation protocol and luteal support with progesterone. Human Reproduction 1996;11:1552‐7. - PubMed
Ho 2008 {published data only}
-
- Ho CH, Chen SU, Peng FS, Chang CY, Yang YS. Luteal support for IVF/ICSI cycles with Crinone 8% (90 mg) twice daily results in higher pregnancy rates than with intramuscular progesterone. Journal of the Chinese Medical Association 2008;71(8):386‐91. - PubMed
Hokenstad 2013 {published data only}
-
- Hokenstad AN, Leonard PH, Morbeck DE, Khan Z, Asante A, Coddington CC. Route of luteal phase support for frozen embryo transfers: a randomized control trial. Reproductive Sciences 2013;20(S3):233A (Abs# F‐141).
Humaidan 2010 {published data only}
-
- Humaidan P, Ejdrup Bredkjaer H, Westergaard LG, Yding Andersen C. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin‐releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertility and Sterility 2010;93(3):847‐54. - PubMed
Humaidan 2013 {published data only}
-
- Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi‐centre studies in IVF patients. Human Reproduction 2013;28(9):2511‐21. - PubMed
Hutchinson‐Williams 1990 {published data only}
-
- Hutchinson‐Williams KA, DeCherney AH, Lavy G, Diamond MP, Naftolin F, Lunenfeld B. Luteal rescue in in vitro fertilization‐embryo transfer. Fertility and Sterility 1990;53:495‐9. - PubMed
Iliodromiti 2013 {published data only}
-
- Iliodromiti S, Blockeel C, Tremellen KP, Fleming R, Tournaye H, Humaidan P, Nelson SM. Consistent high clinical pregnancy rates and low ovarian hyperstimulation syndrome rates in high‐risk patients after GnRH agonist triggering and modified luteal support: a retrospective multicentre study. Human Reproduction 2013;28:2529‐36. - PubMed
Jee 2010 {published data only}
-
- Jee BC, Suh CS, Kim SK, Kim YB, Moon SY. Effects of estradiol supplementation during the luteal phase of in vitro fertilization cycles: a meta‐analysis. Fertility and Sterility 2010;93(2):428‐36. - PubMed
Johnson 1999 {published data only}
-
- Johnson MR, Okokon E, Collins WP, Sharma V, Lightman SL. The effect of human chorionic gonadotropin and pregnancy on the circulating level of relaxin. Journal of Clinical Endocrinology and Metabolism 1999;72:1042‐7. - PubMed
Jung 2010 {published data only}
-
- Jung YH, Kim YY, Kim MH, Cho JD. The best luteal phase support protocol for patients who had E2 levels <1500 pg/ml on the hCG day in a long GnRH agonist cycles. Fertility and Sterility 2010;94 Suppl 1(4):177.
Kahraman 2010 {published data only}
-
- Kahraman S, Karagozoglu SH, Karlikaya G. The efficiency of progesterone vaginal gel versus intramuscular progesterone for luteal phase supplementation in gonadotropin‐releasing hormone antagonist cycles: a prospective clinical trial. Fertility and Sterility 2010;94(2):761‐3. - PubMed
-
- Karagozoglu H, Kahraman S, Karlikaya G, Kavrut M, Ersahin A. The efficiency of vaginal gel vs intramuscular progesterone for luteal phase support in GnRH antagonist cycles: a prospective, randomized trial. Human Reproduction. 2009; Vol. 24 Suppl 1:i109‐10 (Abs #O‐273).
Kaser 2012 {published data only}
-
- Kaser D, Ginsburg E, Missmer S, Correia K, Racowsky C. Intramuscular progesterone versus Crinone 8% vaginal gel for luteal phase replacement in day 3 cryopreserved embryo transfer. Human Reproduction 2012;27:P‐243. - PubMed
-
- Kaser DJ, Ginsburg ES, Missmer SA, Correia KF, Racowsky C. Intramuscular progesterone versus 8% Crinone vaginal gel for luteal phase support for day 3 cryopreserved embryo transfer. Fertility and Sterility 2012;98:1464‐9. - PubMed
Kol 2011 {published data only}
-
- Kol S, Humaidan P, Itskovitz‐Eldor J. GnRH agonist ovulation trigger and hCG‐based, progesterone‐free luteal support: a proof of concept study. Human Reproduction 2011;26:2874‐7. - PubMed
Koper 2008 {published data only}
-
- The Corifollitropin Alfa Dose‐finding Study Group. A randomized dose‐response trial of a single injection of corifollitropin alfa to sustain multifollicular growth during controlled ovarian stimulation. Human Reproduction 2008;23(11):2484‐92. - PubMed
Krause 2006 {published data only}
-
- Krause BT, Ohlinger R. Safety and efficacy of low dose hCG for luteal support after triggering ovulation with a GnRH agonist in cases of polyfollicular development. European Journal of Obstetrics Gynecology and Reproductive Biology 2006;126(1):87‐92. - PubMed
Krischker 1998 {published data only}
-
- Krischker U, Poehl M, Bichler K, Feichtinger W. Different methods of luteal phase support in an in vitro fertilization (IVF) program [abstract]. Fertility and Sterility 1998;70 Suppl 1:327 (Abs # P‐639).
Kwon 2012 {published data only}
-
- Kwon SK, Kim CH, Ahn JW, Lee KH, Chae HD, Kang BM. Effect of intravenous immunoglobulin on pregnancy outcome following IVF/ICSI in infertile patients with endometriosis. Fertility and Sterility 2012;100:S263 (Abs# P‐511).
Kyrou 2011a {published data only}
-
- Kyrou D, Kolibianakis EM, Fatemi HM, Tarlatzi TB, Devroey P, Tarlatzis BC. Increased live birth rates with GnRH agonist addition for luteal support in ICSI/IVF cycles: a systematic review and meta‐analysis. Human Reproduction Update 2011;17:734‐40. - PubMed
Lainas 2012 {published data only}
Lam 2003 {published data only}
-
- Lam PM, Cheung LP, Haines CJ. Early luteal phase progesterone supplementation and IVF‐ET outcome [abstract]. Reproduction 2003;Abstract Series #30:51 (Abs # P2).
Lan 2007 {published data only}
-
- Lan VTN, Tuan P, Canh L, Tuong H, Howles CM. Comparison of the efficacy and tolerability of two formulations of vaginal progesterone for luteal phase support in frozen embryo transfer cycles. Fertility and Sterility 2007;88 Suppl 1:164 (Abs #169).
Lee 2013 {published data only}
-
- Lee VC, Li RH, Ng EH, Yeung WS, Ho PC. Luteal phase support does not improve the clinical pregnancy rate of natural cycle frozen‐thawed embryo transfer: a retrospective analysis. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2013;169:50‐3. - PubMed
Lee 2013a {published data only}
-
- Lee JH, Kim SG, Kim YY, Kim HJ, Lee KH, Park IH, Sun HG. The effect of additional low dose hCG with vaginal progesterone gel in luteal phase of IVF cycles. Human Reproduction 2013;28:323.
Leeton 1985 {published data only}
-
- Leeton J, Trounson A, Jessup D. Support of the luteal phase in in vitro fertilization programs: results of a controlled trial with intramuscular proluton. Journal of In Vitro Fertilization and Embryo Transfer 1985;2:166‐9. - PubMed
Lightman 1999 {published data only}
-
- Lightman A, Kol S, Itskovitz‐Eldor J. A prospective randomized study comparing intramuscular with intravaginal natural progesterone in programmed thaw cycles. Human Reproduction 1999;14:2596‐9. - PubMed
Lin 2013a {published data only}
-
- Lin H, Li Y, Li L, Zhang Q, Wang W, Chen X, Yang D. Effect of delayed initiation of gonadotropin in luteal long protocol on in vitro fertilization. Gynecological Endocrinology 2013;29:846‐50. - PubMed
Liu 2012 {published data only}
Lukaszuk 2005 {published data only}
-
- Lukaszuk K, Liss J, Lukaszuk M, Maj B. Optimization of estradiol supplementation during the luteal phase improves the pregnancy rate in women undergoing in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2005;83(5):1372‐6. - PubMed
Mahadevan 1985 {published data only}
-
- Mahadevan MM, Leader A, Taylor PJ. Effects of low‐dose human chorionic gonadotropin on corpus luteum function after embryo transfer. Journal of In Vitro Fertilization and Embryo Transfer 1985;2:190‐4. - PubMed
Marianowski 2000 {published data only}
-
- Marianowski P, Radwanska E. Intramuscular vs vaginal progesterone for luteal support in cycles of in vitro fertilization. Ginekologia Polska 2000;71:1064‐70. - PubMed
Martins 2010 {published data only}
-
- Martins WdP. Suporte da fase lutea. Femina 2010;38(5):271.
McBain 1987 {published data only}
-
- McBain J C, Clarke G A, Molloy D, Yeates J, Johnston W I H, McKenna M. A randomized trial of progesterone support following ovarian stimulation with clomiphene hMG for IVF and GIFT [abstract]. 5th World Congress on In Vitro Fertilization and Embryo Transfer Abstract Book. 1987:75 (Abs # PP‐126).
Michnova 2011 {published data only}
-
- Michnova L, Rumpikova T, Dostal J. Luteal support in the IVF/ET programme. Ceska Gynekologie / Ceska Lekarska Spolecnost J. Ev. Purkyne 2011;76(2):104‐7. - PubMed
Miller 2013 {published data only}
-
- Miller CE, Zbella E, Webster BW, Doody KJ, Bush MR, Collins MG. Clinical comparison of ovarian stimulation and luteal support agents in patients undergoing GnRH antagonist IVF cycles. Journal of Reproductive Medicine 2013;58:153‐60. - PubMed
Mochtar 1996 {published data only}
-
- Mochtar MH, Hogerzeil HV, Mol BWJ. Progesterone alone versus progesterone combined with HCG as luteal support in GnRHa/HMG induced IVF cycles: a randomized clinical trial. Human Reproduction 1996;11:1602‐5. - PubMed
Moraloglu 2008 {published data only}
-
- Moraloglu O, Kilic S, Karayalcin R, Yuksel B, Tasdemir N, Isik A, et al. Comparison of GnRH agonists and antagonists in normoresponder IVF/ICSI in Turkish female patients. Advances in Therapy 2008;25(3):266‐73. - PubMed
Munoz 2013 {published data only}
-
- Munoz E, Taboas E, Portela S, Aguilar J, Fernandez I, Munoz L, et al. Treatment of luteal phase defects in assisted reproduction. Current Drug Targets 2013;14(8):832‐42. - PubMed
Nader 1988 {published data only}
-
- Nader S, Berkowitz AS, Ochs D, Held B, Winkel CA. Luteal‐phase support in stimulated cycles in an in vitro fertilization/embryo transfer program: progesterone versus human chorionic gonadotropin. Journal of In Vitro Fertilization and Embryo Transfer 1988;5:81‐4. - PubMed
NCT01007851 2006 {published data only}
-
- NCT01007851. Single Dose Gonadotropin‐releasing Hormone (GnRH) Agonist Administration in the Luteal Phase of GnRH Antagonist Stimulated ICSI‐ET Cycles. https://clinicaltrials.gov/ct2/show/NCT01007851 2006. [MEDLINE: ]
Nikkanen 1992 {published data only}
-
- Nikkanen V, Kresanov I, Makinen J, Vuorento T. The effect of luteal support with human chorionic gonadotrophin or progesterone on the daily progesterone profile after different types of ovarian stimulation. Human Reproduction 1992;7:333‐6. - PubMed
Nyboe Andersen 2012 {published data only}
-
- Nyboe Andersen A, Lauritsen MP, Thuesen LL. Withdrawal of progesterone support on the day of positive hCG after IVF/ICSI has no effect on miscarriage rates. Evidence from two large prospective trials. Human Reproduction 2012;27:Abs# P‐486.
Osmanagaoglu 2013 {published data only}
-
- Osmanagaoglu K, Decleer W, Seynhave B, Kolibianakis E, Tarlatzis B, Devroey P. Prospective randomized controlled trial in gnrh antagonist stimulated cycles comparing HCG triggering alone versus HCG triggering associated with gnrh agonist. Fertility and Sterility 2013;100:S1‐S2.
Ozcimen 2004 {published data only}
-
- Ozcimen EE, Ugur M, Ozcimen N, Yilmaz Z. Is luteal phase support with hCG or vaginal micronised progesterone beneficial in non‐IVF gonadotropin induction of ovulation?. Fertility and Sterility 2004;82 Suppl 2:142.
Papanikolaou 2010 {published data only}
-
- Papanikolaou E, Verpoest W, Fatemi H, Polyzos N, Humaidan P, Tarlatzis B, et al. Recombinant LH as luteal supplementation method after agonist triggering in IVF. A proof of concept study [abstract]. Human Reproduction. 2010; Vol. 25:i167‐8 (Abs #P‐134).
-
- Papanikolaou EG, Fatemi H, Kyrou D, Polyzos NP, Humaidan P, Tarlatzis B, et al. Higher birth rate after recombinant hCG triggering compared with urinary‐derived hCG in single‐blastocyst IVF antagonist cycles: a randomized controlled trial. Fertility and Sterility 2010;94(7):2902‐4. - PubMed
Papanikolaou 2011 {published data only}
-
- Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H. A novel method of luteal supplementation with recombinant luteinizing hormone when a gonadotropin‐releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: a randomized prospective proof of concept study. Fertility and Sterility 2011;95:1174‐7. - PubMed
Paredes 2004 {published data only}
-
- Paredes Chavez FC, Barros Delgadillo JC, Ochoa Rueda SS, Barroso Villa G, et al. [Papel de los estrógenos en el soporte de la fase lútea en ciclos de fertilización in vitro con transferencia de embriones]. Ginecología y Obstetricia de México 2004;72(12):645‐55. - PubMed
Pirard 2005 {published data only}
-
- Pirard C, Donnez J, Loumaye E. GnRH agonist as novel luteal support: results of a randomized, parallel group, feasibility study using intranasal administration of buserelin. Human Reproduction 2005;20(7):1798‐804. - PubMed
Pirard 2006 {published data only}
-
- Pirard C, Donnez J, Loumaye E. GnRH agonist as luteal phase support in assisted reproduction technique cycles: results of a pilot study. Human Reproduction 2006;21(7):1894‐900. - PubMed
Polson 1992 {published data only}
-
- Polson DW, Rogers PAW, Krapez JA, Leeton JF. Vaginal progesterone as luteal phase support in an IVF/GIFT programme. European Journal of Obstetrics Gynecology and Reproductive Biology 1992;46(1):35‐8. - PubMed
Priyadharshini 2013 {published data only}
-
- Priyadharshini M, Sathya B, Varma T. Dydrogesterone is not inferior to natural progesterone for luteal support in ART (IVF/ICSI) cycles. BJOG: An International Journal of Obstetrics and Gynaecology 2013;120:214.
Propst 2012 {published data only}
-
- Propst AM, Thoppil JJ, Groll JM, Frattarelli JL, Robinson RD, Retzloff MG. A single pre‐ovulatory IUI at 12 hours after hCG trigger is comparable to a traditional IUI at 36 hours. Fertility and Sterility 2012;98(Suppl 1):S85‐S86 (Abs# O‐288).
Santibanez 2014 {published data only}
-
- Santibanez A, Garcia J, Pashkova O, Colin O, Castellanos G, Sanchez AP, Jara JF. Effect of intrauterine injection of human chorionic gonadotropin before embryo transfer on clinical pregnancy rates from in vitro fertilisation cycles: a prospective study. Reproductive Biology and Endocrinology 2014;12:9‐13. [DOI: 10.1186/1477-7827-12-9] - DOI - PMC - PubMed
Satir 2013 {published data only}
Schwarzler 2003 {published data only}
-
- Schwarzler P, Abendstein BJ, Klingler A, Kreuzer E, Rjosk HK. Prevention of severe ovarian hyperstimulation syndrome (OHSS) in IVF patients by steroidal ovarian suppression ‐ A prospective randomized study. Human Fertility 2003;6(3):125‐9. - PubMed
Shamma 1992 {published data only}
-
- Shamma F, Haj‐Hassan L, Penzias A, Gutmann J, Leykin L, Jones E. Luteal phase support in in vitro fertilization embryo transfer (IVF‐ET) ‐ a prospective randomized trial [abstract]. American Fertility Society 48th Annual Meeting Abstract Book. 1992:S140‐1 (Abs # P‐074).
Silverberg 2010 {published data only}
-
- Silverberg K, Vaughn TC, Hansard L, Burger N, Minter T. Progesterone vaginal gel vs. intramuscular progesterone in oil for luteal support in IVF: a large, prospective trial [abstract]. Fertility and Sterility. 2010; Vol. Suppl 1:21 (Abs # O‐69). - PubMed
Simunic 2007 {published data only}
-
- Simunic V, Tomic V, Tomic J, Nizic D. Comparative study of the efficacy and tolerability of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support. Fertility and Sterility 2007;87(1):83‐7. - PubMed
Singh 2010 {published data only}
-
- Singh T, Majumdar A. Supplementation of gonadotrophin releasing hormone (GnRH) agonist during the luteal phase improves the pregnancy outcome in intrauterine insemination (IUI) cycles, when compared with human chorionic gonadotrophin (hCG). Journal fur Reproduktionsmedizin und Endokrinologie. Munich, Germany, 2010:277 (Abs 15‐7).
Smith 1989 {published data only}
Smitz 1988 {published data only}
-
- Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, et al. The luteal phase and early pregnancy after combined GnRH‐agonist/HMG treatment for superovulation in IVF or GIFT. Human Reproduction 1988;3(5):585‐90. - PubMed
Smitz 1992 {published data only}
-
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem A. A prospective randomized comparison of intramuscular or intravaginal natural progesterone as a luteal phase and early pregnancy supplement. Human Reproduction 1992;7:168‐75. - PubMed
-
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem AC. A randomized prospective study comparing supplementation of the luteal phase and early pregnancy by natural progesterone administered by intramuscular or vaginal route [Etude prospective randomisee comparant la supplementation de la phase luteale et de la grossesse debutante par la progesterone naturelle administree par voie intra‐musculaire ou vaginale]. Revue Francaise de Gynecologie et d Obstetrique 1992;87(10):507‐16. - PubMed
Smitz 1993 {published data only}
-
- Smitz J, Bourgain C, Waesgerghe L, Camus M, Devroey P, Steirteghem AC. A prospective randomized study on oestradiol valerate supplementation in addition to intravaginal micronized progesterone in buserelin and HMG induced superovulation. Human Reproduction 1993;8:40‐5. - PubMed
Sordal 1993 {published data only}
-
- Sordal T, Kahn J A, Sunde A, Düring V, Molne K. A prospective randomized study comparing natural progesterone administered intramuscularly and vaginal micronized progesterone for luteal support [abstract]. Human Reproduction 1993;8 Suppl 1:39 (Abs # 99).
Stadtmauer 2009 {published data only}
-
- Stadtmauer L, Harrison DD, Boyd J, Bocca S, Oehninger S. Pilot study evaluating a progesterone vaginal ring for luteal‐phase replacement in donor oocyte recipients. Fertility and Sterility 2009;92(5):1600‐5. - PubMed
Stovall 1998 {published data only}
-
- Stovall DW, Voorhis BJ, Sparks AE, Adams LM, Syrop CH. Selective early elimination of luteal support in assisted reproduction cycles using a gonadotropin‐releasing hormone agonist during ovarian stimulation. Fertility & Sterility 1998;70(6):1056‐62. - PubMed
Tay 2003 {published data only}
-
- Tay PY, Lenton EA. Inhibition of progesterone secretion by oestradiol administered in the luteal phase of assisted conception cycles. Medical Journal of Malaysia 2003;58(2):187‐95. - PubMed
Tomic 2011 {published data only}
-
- Tomic V, Tomic J, Klaic DZ. Oral micronized progesterone combined with vaginal progesterone gel for luteal support. Gynecological Endocrinology 2011;27:1010‐3. - PubMed
Trounson 1986 {published data only}
-
- Trounson A, Howlett D, Rogers P, Hoppen HO. The effect of progesterone supplementation around the time of oocyte recovery in patients superovulated for in vitro fertilization. Fertility and Sterility 1986;45:532‐5. - PubMed
Unfer 2004 {published data only}
-
- Unfer V, Casini M, Costabile L, Gerli S, Baldini D, Renzo GC. 17 alpha‐hydroxyprogesterone caproate versus intravaginal progesterone in IVF‐embryo transfer cycles: a prospective randomized study. Reproductive BioMedicine Online 2004;9(1):17‐21. - PubMed
Unfer 2004a {published data only}
-
- Unfer V, Casini ML, Gerli S, Costabile L, Mignosa M, Renzo GC. Phytoestrogens may improve the pregnancy rate in in vitro fertilization‐embryo transfer cycles: a prospective, controlled, randomized trial. Fertility and Sterility 2004;82(6):1509‐13. - PubMed
Vaisbuch 2012 {published data only}
-
- Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence‐based medicine translated to clinical practice? A worldwide web‐based survey. Reproductive Biomedicine Online 2012;25(2):139‐45. - PubMed
Valentino 2004 {published data only}
-
- Valentino V, Artini PG, Ruggiero M, Parisen Toldin MR, Cristello F, et al. A randomised comparison of effects and patient inconvenience of two progesterone supplementation used in in vitro fertilisation cycles. Gynaecological Endocrinology 2004;18 Suppl 1:358 (Abs # P‐170).
van Steirteghem 1988 {published data only}
-
- Steirteghem AC, Smitz J, Camus M, Waesberghe L, Deschacht J, Khan I, et al. The luteal phase after in‐vitro fertilization and related procedures. Human Reproduction 1988;3:161‐4. - PubMed
Var 2011 {published data only}
-
- Var T, Aysin Tonguc E, Doganay M, Gulerman C, Gungor T, Mollamahmutoglu L. A comparison of the effects of three different luteal phase support protocols on in vitro fertilization outcomes: a randomized clinical trial. Fertility and Sterility 2011;95:985‐9. - PubMed
Wang 2009 {published data only}
-
- Wang LJ, Huang FJ, Kung FT, Lin PY, Chang SY, Lan KC. Comparison of the efficacy of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support in blastocyst stage embryo transfers. Taiwanese Journal of Obstetrics & Gynecology 2009;48(4):375‐9. - PubMed
Wilcox 2001 {published data only}
-
- Wilcox J, Nelson JR, Potter D, Frederick J, Feinman M, Batzofin J. Comparison of different luteal phase support protocols for frozen embryo transfer (FET) [abstract]. Fertility and Sterility 2001;76 Suppl 1:124 (Abs # P‐37).
Yazici 2014 {published data only}
-
- Yazici G, Savas A, Tasdelen B, Dilek S. Role of luteal phase support on gonadotropin ovulation induction cycles in patients with polycystic ovary syndrome. Journal of Reproductive Medicine 2014;59:25‐30. - PubMed
Ye 2009 {published data only}
Yigit 2002 {published data only}
-
- Yigit N, Halicigil C, Basaran M, Aksu T, Yarali H. Crinone and i.m. progesterone yield comparable pregnancy rates following ICSI and embryo transfer [title only]. Human Reproduction 2002;17(Abstract Book 1):201 (Abs # R‐624).
Yovich 1984 {published data only}
-
- Yovich JL, Stanger JD, Yovich JM, Tuvik AI. Assessment and hormonal treatment of the luteal phase of in vitro fertilization cycles. Australian & New Zealand Journal of Obstetrics & Gynaecology 1984;24:125‐30. - PubMed
Yovich 1985 {published data only}
-
- Yovich JL, McColm SC, Yovich JM, Matson PL. Early luteal serum progesterone concentrations are higher in pregnancy cycles. Fertility and Sterility 1985;44:185‐9. - PubMed
Yovich 1991 {published data only}
-
- Yovich JL, Rohini Edirisinghe W, Cummins JM. Evaluation of luteal support therapy in a randomized controlled study within a gamete intrafallopian transfer program. Fertility and Sterility 1991;55:131‐9. - PubMed
References to studies awaiting assessment
Pirard 2015 {published data only}
Tomic 2015 {published data only}
-
- Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. European journal of obstetrics, gynecology, and reproductive biology 2015;186:49‐53. [PUBMED: 25622239] - PubMed
Zafardoust 2015 {published data only}
References to ongoing studies
EUCTR2012‐002215‐26‐BE 2013 {published data only}
-
- EUCTR 2012‐002215‐26‐BE. A study to compare if 30mg of oral Dydrogesterone is as good, tolerable and safe as 600mg of intravaginal capsules for luteal support in IVF pregnancies. This study will be conducted at several study sites and neither the patient or the doctor will know which of the two different treatments a patient will receive. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2012‐00221... 2013.
EUCTR2013‐001105‐81‐2013 {published data only}
-
- EUCTR2013‐001105‐81‐2013. Randomised clinical trial comparing highly purified FSH formulation (Fostimon®) and recombinant FSH (Gonal‐F®) in GnRH‐antagonist controlled ovarian hyperstimulation cycles. EU Clinical Trials Registry.
IRCT201402191141N18 2015 {published data only}
-
- IRCT201402191141N18. Subcutaneous progesterone versus vaginal suppository for luteal phase support in assisted reproductive technology cycles in patients referred to Royan Institute. https://translate.google.co.nz/translate?hl=en&sl=fa&u=http://ww... 2015.
IRCT2014030916912N1 2014 {published data only}
-
- IRCT2014030916912N1. Effect of administration GNRH agonist(Triptrolin) on clinical pregnancy in ART. https://translate.google.co.nz/translate?hl=en&sl=fa&u=http://ww... 2014.
IRCT2014071212494N2 2014 {published data only}
-
- IRCT2014071212494N2. Effect of progesterone on pregnancy rate of assisted reproduction. http://www.irct.ir/searchtotal.php?&page=706 2014.
NCT00490308 2007 {published data only}
-
- NCT00490308. The Influence of Estradiol Supplementation During the Luteal in Patients Undergoing IVF Treatment. https://clinicaltrials.gov/ct2/show/NCT00490308 2007. [MEDLINE: ]
NCT01081652 {published data only}
-
- NCT01081652. A Study Using Micronised Progesterone (Crinone® 8%) in the Luteal Phase Support of Women Undergoing in Vitro Fertilisation (IVF) and Embryo Transfer (ET). https://www.clinicaltrials.gov/ct2/show/NCT01081652 2014.
NCT01237535 {published data only}
-
- NCT01237535. Luteal Phase Support With Progesterone Versus Estrogen and Progesterone on Pregnancy Rates. https://clinicaltrials.gov/ct2/show/NCT01237535 2010.
NCT01504139 2012 {published data only}
-
- NCT01504139. The Luteal Phase After GnRHa Trigger ‐ a Proof of Concept Study. http://www.ncbi.nlm.nih.gov/pubmed/26209535 2012. [MEDLINE: ]
NCT01638026 2012 {published data only}
-
- NCT01638026. Final Oocyte Maturation Via Administration of GnRH Agonists Followed By Luteal Support With hCG. https://clinicaltrials.gov/ct2/show/NCT01638026 2012. [MEDLINE: ]
NCT01790282 2013 {published data only}
-
- NCT01790282. Is Adding E2 to P4 Luteal Support In High Responder Long Gn‐RH Agonist ICSI Cycles Detrimental to Outcome? RCT. https://clinicaltrials.gov/ct2/show/NCT01790282 2013.
NCT01850030 {published data only}
-
- NCT01850030. A Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg Daily Versus Intravaginal Micronized Progesterone Capsules 600 mg Daily for Luteal Support in In‐Vitro Fertilization. https://clinicaltrials.gov/ct2/show/NCT01850030 2015.
NCT01863680 2013 {published data only}
-
- NCT01863680. Phase 3 Trial to Evaluate the Efficacy and Safety of COL‐1620 Vaginal Progesterone Gel. https://clinicaltrials.gov/ct2/show/NCT01863680 2013.
NCT01980680 2013 {published data only}
-
- NCT01980680. The Exogenous Progesterone Free Luteal Phase After GnRHa Trigger ‐ a Pilot Study in Normo‐responder IVF Patients. https://clinicaltrials.gov/ct2/show/NCT01980680 2013.
NCT02053779 2014 {published data only}
-
- NCT02053779. Luteal Phase Plus GnRH‐agonist After GnRH‐agonist Triggering Combined With Low Dose HCG in IVF (LPGGTHI). https://clinicaltrials.gov/ct2/show/NCT02053779 2014.
NCT02114645 2014 {published data only}
-
- NCT02114645. The Effect of GnRH Agonist Administered in the Luteal Phase on ART Cycle Outcomes. https://clinicaltrials.gov/ct2/show/NCT02114645 2014.
NCT02262416 2014 {published data only}
-
- NCT02262416. GnRH Agonist and Progesterone Versus Progesterone Only for Luteal Phase Support in Antagonist Cycles. https://clinicaltrials.gov/ct2/show/NCT02262416 2014.
NCT02312076 2014 {published data only}
-
- NCT02312076. GnRHa for Luteal Phase Support in Long GnRHa Protocol Cycles. https://clinicaltrials.gov/ct2/show/NCT02312076 2014.
NCT02312089 2014 {published data only}
-
- NCT02312089. GnRHa for Luteal Phase Support in GnRH Antagonist Protocol Cycles. https://clinicaltrials.gov/ct2/show/NCT02312089 2014.
NCT02316626 2014 {published data only}
-
- NCT02316626. Subcutaneous Progesterone Versus Vaginal Progesterone Gel for Luteal Phase Support. https://clinicaltrials.gov/ct2/show/NCT02316626 2014. - PubMed
NCT02357654 2015 {published data only}
-
- NCT02357654. GnRH for Luteal Support in IVF/ICSI/FET Cycles. https://clinicaltrials.gov/ct2/show/NCT02357654 2015. [MEDLINE: ]
NCT02491437 {published data only}
-
- NCT02491437. A Randomized, Open‐label, Two‐arm, Multicenter Study Comparing the Efficacy, Safety and Tolerability of Oral Dydrogesterone 30 mg Daily Versus Crinone 8% Intravaginal Progesterone Gel 90 mg Daily for Luteal Support in In‐Vitro Fertilization (LOTUS II). https://clinicaltrials.gov/ct2/show/NCT02491437 2015.
Additional references
Balasch 2004
-
- Balasch J. The role of FSH and LH in ovulation induction: current concepts and the contribution of recombinant gonadotropins. In: Gardner DK, Weissman A, Howles and Shoham V editor(s). Textbook of Assisted Reproductive Techniques. Laboratory and Clinical Perspectives. 2nd Edition. London and New York: Taylor & Francis, 2004:541‐65.
CDC 2009
-
- Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology 2007. Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2009.
Chan 2003
-
- Chan CC, Ng EH, Chan MM, Tang OS, Lau EY, Yeung WS, et al. Bioavailability of hCG after intramuscular or subcutaneous injection in obese and non‐obese women. Human Reproduction 2003;18(11):2294‐7. - PubMed
Choavaratana 2004
-
- Choavaratana R, Manoch D. Efficacy of oral micronized progesterone when applied via vaginal route. Journal of the Medical Association of Thailand 2004;87(5):455‐8. - PubMed
de Mouzon 2010
-
- Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, et al. Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Human Reproduction 2010;25(8):1851‐62. - PubMed
Edwards 1980
-
- Edwards RG, Steptoe PC, Purdy JM. Establishing full‐term human pregnancies using cleaving embryos grown in vitro. British Journal of Obstetrics and Gynaecology 1980;87(9):737‐56. - PubMed
Farquhar 2010
-
- Farquhar C, Roberts H. Introduction to Obstetrics and Gynaecology. Third Edition. Auckland: Department of Obstetrics & Gynaecology, The University of Auckland, 2010.
Fatemi 2009
-
- Fatemi HM. The luteal phase after 3 decades of IVF: what do we know?. Reproductive Biomedicine Online 2009;19(4):4331. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kerin 1981
-
- Kerin JF, Broom TJ, Ralph MM, Edmonds DK, Warnes GM, Jeffrey R, et al. Human luteal phase function following oocyte aspiration from the immediately preovular graafian follicle of spontaneous ovular cycles. British Journal of Obstetrics and Gynaecology 1981;88(10):1021‐8. - PubMed
Macaldowie 2014
-
- Macaldowie A, Wang YA, Chughtai AA, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2012. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales. 2014.
Mannaerts 1998
-
- Mannaerts BM, Geurts TB, Odink J. A randomized three‐way cross‐over study in healthy pituitary‐suppressed women to compare the bioavailability of human chorionic gonadotrophin (Pregnyl) after intramuscular and subcutaneous administration. Human Reproduction 1998;13(6):1461‐4. - PubMed
Messinis 2009
-
- Messinis IE, Messini CI, Dafopoulos K. Luteal‐phase endocrinology. Reproductive Biomedicine Online 2009;19(4):4314. - PubMed
Miyake 1979
-
- Miyake A, Aono T, Kinugasa T, Tanizawa O, Kurachi K. Suppression of serum levels of luteinizing hormone by short‐ and long‐loop negative feedback in ovariectomized women. Journal of Endocrinology 1979;80(3):353‐6. - PubMed
Mochtar 2007
Pabuccu 2005
-
- Pabuccu R, Akar ME. Luteal phase support in assisted reproductive technology. Current Opinion in Obstetrics and Gynecology 2005;17(3):277‐81. - PubMed
Saal 1991
-
- Saal W, Glowania HJ, Hengst W, Happ J. Pharmacodynamics and pharmacokinetics after subcutaneous and intramuscular injection of human chorionic gonadotropin. Fertility and Sterility 1991;56(2):225‐9. - PubMed
Schindler 2009
-
- Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas 2009;65(1):S3‐11. - PubMed
Smitz 1992a
-
- Smitz J, Erard P, Camus M, Devroey P, Tournaye H, Wisanto A, et al. Pituitary gonadotrophin secretory capacity during the luteal phase in superovulation using GnRH‐agonists and HMG in a desensitization or flare‐up protocol. Human Reproduction 1992;7(9):1225‐9. - PubMed
Tavaniotou 2003
-
- Tavaniotou A, Devroey P. Effect of human chorionic gonadotropin on luteal luteinizing hormone concentrations in natural cycles. Fertility and Sterility 2003;80(3):654‐5. - PubMed
Tesarik 2004
-
- Tesarik J, Hazout A, Mendoza C. Enhancement of embryo developmental potential by a single administration of GnRH agonist at the time of implantation. Human Reproduction 2004;19(5):1176‐80. - PubMed
Wikland 1995
-
- Wikland M, Borg J, Forsberg AS, Jakobsson AH, Svalander P, Waldenstrom U. Human chorionic‐gonadotropin self‐administered by the subcutaneous route to induce oocyte maturation in an in‐vitro fertilization and embryo‐transfer program. Human Reproduction 1995;10(7):1667‐70. - PubMed
References to other published versions of this review
Daya 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

