Multiple microRNAs within the 14q32 cluster target the mRNAs of major type 1 diabetes autoantigens IA-2, IA-2β, and GAD65
- PMID: 26148972
- PMCID: PMC4566937
- DOI: 10.1096/fj.15-273649
Multiple microRNAs within the 14q32 cluster target the mRNAs of major type 1 diabetes autoantigens IA-2, IA-2β, and GAD65
Abstract
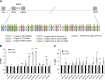
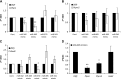
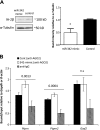
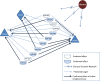
Islet antigen (IA)-2, IA-2β, and glutamate decarboxylase (GAD65) are major autoantigens in type 1 diabetes (T1D). Autoantibodies to these autoantigens appear years before disease onset and are widely used as predictive markers. Little is known, however, about what regulates the expression of these autoantigens. The present experiments were initiated to test the hypothesis that microRNAs (miRNAs) can target and affect the levels of these autoantigens. Bioinformatics was used to identify miRNAs predicted to target the mRNAs coding IA-2, IA-2β, and GAD65. RNA interference for the miRNA processing enzyme Dicer1 and individual miRNA mimics and inhibitors were used to confirm the effect in mouse islets and MIN6 cells. We show that the imprinted 14q32 miRNA cluster contains 56 miRNAs, 32 of which are predicted to target the mRNAs of T1D autoantigens and 12 of which are glucose-sensitive. Using miRNA mimics and inhibitors, we confirmed that at least 7 of these miRNAs modulate the mRNA levels of the T1D autoantigens. Dicer1 knockdown significantly reduced the mRNA levels of all 3 autoantigens, further confirming the importance of miRNAs in this regulation. We conclude that miRNAs are involved in regulating the expression of the major T1D autoantigens.
Keywords: autoimmune diabetes; glucose-sensitive; miR-342; miRNA.
© FASEB.
Figures

References
-
- Pihoker C., Gilliam L. K., Hampe C. S., Lernmark A. (2005) Autoantibodies in diabetes. Diabetes 54(Suppl 2), S52–S61 - PubMed
-
- Kubosaki A., Nakamura S., Notkins A. L. (2005) Dense core vesicle proteins IA-2 and IA-2beta: metabolic alterations in double knockout mice. Diabetes 54(Suppl 2), S46–S51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

