Synthesis of magnetic metal-organic framework (MOF) for efficient removal of organic dyes from water
- PMID: 26149818
- PMCID: PMC4493573
- DOI: 10.1038/srep11849
Synthesis of magnetic metal-organic framework (MOF) for efficient removal of organic dyes from water
Abstract
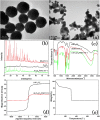
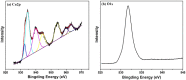
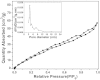
A novel, simple and efficient strategy for fabricating a magnetic metal-organic framework (MOF) as sorbent to remove organic compounds from simulated water samples is presented and tested for removal of methylene blue (MB) as an example. The novel adsorbents combine advantages of MOFs and magnetic nanoparticles and possess large capacity, low cost, rapid removal and easy separation of the solid phase, which makes it an excellent sorbent for treatment of wastewaters. The resulting magnetic MOFs composites (also known as MFCs) have large surface areas (79.52 m(2) g(-1)), excellent magnetic response (14.89 emu g(-1)), and large mesopore volume (0.09 cm(3) g(-1)), as well as good chemical inertness and mechanical stability. Adsorption was not drastically affected by pH, suggesting π-π stacking interaction and/or hydrophobic interactions between MB and MFCs. Kinetic parameters followed pseudo-second-order kinetics and adsorption was described by the Freundlich isotherm. Adsorption capacity was 84 mg MB g(-1) at an initial MB concentration of 30 mg L(-1), which increased to 245 mg g(-1) when the initial MB concentration was 300 mg L(-1). This capacity was much greater than most other adsorbents reported in the literature. In addition, MFC adsorbents possess excellent reusability, being effective after at least five consecutive cycles.
Figures


References
-
- Sun L. S. & Luo W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: characterization, equilibrium, and kinetic studies. Bioresour . Technol. 140, 406–413 (2013). - PubMed
-
- Yao Y., Xu F., Chen M., Xu Z. & Zhu Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 101, 3040–3046 (2010). - PubMed
-
- Auta M. & Hameed B. H. Chitosan–clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J. 237, 352–361 (2014).
-
- Auta M. & Hameed B. H. Acid modified local clay beads as effective low-cost adsorbent for dynamic adsorption of methylene blue. J. Ind. Eng. Chem. 19, 1153–1161 (2013).
-
- Liu T. et al. Adsorption of methylene blue from aqueous solution by grapheme. Colloid. Surface. B. 90, 197–203 (2012). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

