Acoustic tweezers for studying intracellular calcium signaling in SKBR-3 human breast cancer cells
- PMID: 26150401
- PMCID: PMC4857610
- DOI: 10.1016/j.ultras.2015.06.017
Acoustic tweezers for studying intracellular calcium signaling in SKBR-3 human breast cancer cells
Abstract
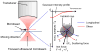
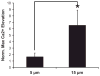
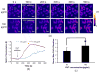
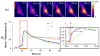
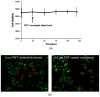
Extracellular matrix proteins such as fibronectin (FNT) play crucial roles in cell proliferation, adhesion, and migration. For better understanding of these associated cellular activities, various microscopic manipulation tools have been used to study their intracellular signaling pathways. Recently, it has appeared that acoustic tweezers may possess similar capabilities in the study. Therefore, we here demonstrate that our newly developed acoustic tweezers with a high-frequency lithium niobate ultrasonic transducer have potentials to study intracellular calcium signaling by FNT-binding to human breast cancer cells (SKBR-3). It is found that intracellular calcium elevations in SKBR-3 cells, initially occurring on the microbead-contacted spot and then eventually spreading over the entire cell, are elicited by attaching an acoustically trapped FNT-coated microbead. Interestingly, they are suppressed by either extracellular calcium elimination or phospholipase C (PLC) inhibition. Hence, this suggests that our acoustic tweezers may serve as an alternative tool in the study of intracellular signaling by FNT-binding activities.
Keywords: Acoustic tweezers; Fibronectin; High-frequency ultrasound transducer; Intracellular calcium elevation; SKBR-3 breast cancer cells.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Cell membrane deformation induced by a fibronectin-coated polystyrene microbead in a 200-MHz acoustic trap.IEEE Trans Ultrason Ferroelectr Freq Control. 2014 Mar;61(3):399-406. doi: 10.1109/TUFFC.2014.2925. IEEE Trans Ultrason Ferroelectr Freq Control. 2014. PMID: 24569245 Free PMC article.
-
Ultrahigh frequency lensless ultrasonic transducers for acoustic tweezers application.Biotechnol Bioeng. 2013 Mar;110(3):881-6. doi: 10.1002/bit.24735. Epub 2012 Oct 16. Biotechnol Bioeng. 2013. PMID: 23042219 Free PMC article.
-
An adjustable multi-scale single beam acoustic tweezers based on ultrahigh frequency ultrasonic transducer.Biotechnol Bioeng. 2017 Nov;114(11):2637-2647. doi: 10.1002/bit.26365. Epub 2017 Jul 18. Biotechnol Bioeng. 2017. PMID: 28654158 Free PMC article.
-
Single-Beam Acoustic Tweezers for Cell Biology: Molecular to In Vivo Level.IEEE Trans Ultrason Ferroelectr Freq Control. 2024 Oct;71(10):1269-1288. doi: 10.1109/TUFFC.2024.3456083. Epub 2024 Oct 10. IEEE Trans Ultrason Ferroelectr Freq Control. 2024. PMID: 39250365 Review.
-
Survey on indirect optical manipulation of cells, nucleic acids, and motor proteins.J Biomed Opt. 2011 May;16(5):051302. doi: 10.1117/1.3579200. J Biomed Opt. 2011. PMID: 21639562 Review.
Cited by
-
Automated cell-type classification combining dilated convolutional neural networks with label-free acoustic sensing.Sci Rep. 2022 Nov 18;12(1):19873. doi: 10.1038/s41598-022-22075-6. Sci Rep. 2022. PMID: 36400803 Free PMC article.
-
Manipulation and Mechanical Deformation of Leukemia Cells by High-Frequency Ultrasound Single Beam.IEEE Trans Ultrason Ferroelectr Freq Control. 2022 Jun;69(6):1889-1897. doi: 10.1109/TUFFC.2022.3170074. Epub 2022 May 26. IEEE Trans Ultrason Ferroelectr Freq Control. 2022. PMID: 35468061 Free PMC article.
-
Label-free analysis of the characteristics of a single cell trapped by acoustic tweezers.Sci Rep. 2017 Oct 26;7(1):14092. doi: 10.1038/s41598-017-14572-w. Sci Rep. 2017. PMID: 29074938 Free PMC article.
-
Integrin Antibody Decreases Deformability of Patient-Derived Pre-B Acute Lymphocytic Leukemia Cells as Measured by High-Frequency Acoustic Tweezers.J Ultrasound Med. 2020 Mar;39(3):589-595. doi: 10.1002/jum.15139. Epub 2019 Oct 21. J Ultrasound Med. 2020. PMID: 31633840 Free PMC article.
-
Automated estimation of cancer cell deformability with machine learning and acoustic trapping.Sci Rep. 2022 Apr 27;12(1):6891. doi: 10.1038/s41598-022-10882-w. Sci Rep. 2022. PMID: 35477742 Free PMC article.
References
-
- Bychkov SM. Fibronectins (review) Vopr Med Khim. 1983;29:2–15. - PubMed
-
- Hynes RO. Fibronectins. Sci Am. 1986;254:42–51. - PubMed
-
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. - PubMed
-
- Rock MT, Brooks WH, Roszman TL. Calcium-dependent signaling pathways in T cells. Potential role of calpain, protein tyrosine phosphatase 1b, and p130Cas in integrin-mediated signaling events. J Biol Chem. 1997;272:33377–33383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

