Host and Environmental Factors Modulate the Exposure of Free-Ranging and Farmed Red Deer (Cervus elaphus) to Coxiella burnetii
- PMID: 26150466
- PMCID: PMC4542230
- DOI: 10.1128/AEM.01433-15
Host and Environmental Factors Modulate the Exposure of Free-Ranging and Farmed Red Deer (Cervus elaphus) to Coxiella burnetii
Abstract
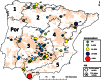
The control of multihost pathogens, such as Coxiella burnetii, should rely on accurate information about the roles played by the main hosts. We aimed to determine the involvement of the red deer (Cervus elaphus) in the ecology of C. burnetii. We predicted that red deer populations from broad geographic areas within a European context would be exposed to C. burnetii, and therefore, we hypothesized that a series of factors would modulate the exposure of red deer to C. burnetii. To test this hypothesis, we designed a retrospective survey of 47 Iberian red deer populations from which 1,751 serum samples and 489 spleen samples were collected. Sera were analyzed by enzyme-linked immunosorbent assays (ELISA) in order to estimate exposure to C. burnetii, and spleen samples were analyzed by PCR in order to estimate the prevalence of systemic infections. Thereafter, we gathered 23 variables-within environmental, host, and management factors-potentially modulating the risk of exposure of deer to C. burnetii, and we performed multivariate statistical analyses to identify the main risk factors. Twenty-three populations were seropositive (48.9%), and C. burnetii DNA in the spleen was detected in 50% of the populations analyzed. The statistical analyses reflect the complexity of C. burnetii ecology and suggest that although red deer may maintain the circulation of C. burnetii without third species, the most frequent scenario probably includes other wild and domestic host species. These findings, taken together with previous evidence of C. burnetii shedding by naturally infected red deer, point at this wild ungulate as a true reservoir for C. burnetii and an important node in the life cycle of C. burnetii, at least in the Iberian Peninsula.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Serological survey of Coxiella burnetii at the wildlife-livestock interface in the Eastern Pyrenees, Spain.Acta Vet Scand. 2016 Apr 27;58:26. doi: 10.1186/s13028-016-0209-4. Acta Vet Scand. 2016. PMID: 27121001 Free PMC article.
-
Prevalence of Coxiella burnetti infection in wild and farmed ungulates.Vet Microbiol. 2008 Jan 1;126(1-3):282-6. doi: 10.1016/j.vetmic.2007.06.020. Epub 2007 Jun 28. Vet Microbiol. 2008. PMID: 17669603
-
Coxiella burnetii Shedding by Farmed Red Deer (Cervus elaphus).Transbound Emerg Dis. 2015 Oct;62(5):572-4. doi: 10.1111/tbed.12179. Epub 2013 Oct 15. Transbound Emerg Dis. 2015. PMID: 24127840
-
Coxiella burnetii in wild mammals: A systematic review.Transbound Emerg Dis. 2019 Mar;66(2):662-671. doi: 10.1111/tbed.13085. Epub 2018 Dec 14. Transbound Emerg Dis. 2019. PMID: 30506629
-
The relevance of the wild reservoir in zoonotic multi-host pathogens: The links between Iberian wild mammals and Coxiella burnetii.Transbound Emerg Dis. 2022 Nov;69(6):3868-3880. doi: 10.1111/tbed.14758. Epub 2022 Nov 26. Transbound Emerg Dis. 2022. PMID: 36335588 Review.
Cited by
-
Estimating the Efficacy of a Commercial Phase I Inactivated Vaccine in Decreasing the Prevalence of Coxiella burnetii Infection and Shedding in Red Deer (Cervus elaphus).Front Vet Sci. 2017 Dec 6;4:208. doi: 10.3389/fvets.2017.00208. eCollection 2017. Front Vet Sci. 2017. PMID: 29270411 Free PMC article.
-
Investigating the Role of Micromammals in the Ecology of Coxiella burnetii in Spain.Animals (Basel). 2021 Mar 2;11(3):654. doi: 10.3390/ani11030654. Animals (Basel). 2021. PMID: 33801164 Free PMC article.
-
Artificial Intelligence Models for Zoonotic Pathogens: A Survey.Microorganisms. 2022 Sep 27;10(10):1911. doi: 10.3390/microorganisms10101911. Microorganisms. 2022. PMID: 36296187 Free PMC article. Review.
-
Seroepidemiology of Coxiella burnetii in Domestic and Wild Ruminant Species in Southern Spain.Animals (Basel). 2024 Oct 24;14(21):3072. doi: 10.3390/ani14213072. Animals (Basel). 2024. PMID: 39518795 Free PMC article.
-
Molecular identification of tick-borne pathogens in ticks collected from dogs and small ruminants from Greece.Exp Appl Acarol. 2018 Apr;74(4):443-453. doi: 10.1007/s10493-018-0237-z. Epub 2018 Mar 7. Exp Appl Acarol. 2018. PMID: 29516380
References
-
- European Food Safety Authority (EFSA). 2010. Panel on Animal Health and Welfare (AHAW). Scientific opinion on Q fever. EFSA J 8:1595. doi:10.2903/j.efsa.2010.1595. - DOI
-
- Ruiz-Fons F. 2012. Coxiella burnetii infection, p 409–412. In Gavier-Wid́en D, Duff JP, Meredith A (ed), Infectious diseases of wild mammals and birds in Europe, 1st ed Wiley-Blackwell, Chichester, United Kingdom.
-
- Georgiev M, Afonso A, Neubauer H, Needham H, Thiéry R, Rodolakis A, Roest HJ, Stärk KD, Stegeman JA, Vellema P, van der Hoek W, More SJ. 2013. Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill 18(8):pi=20407 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

