Reticular dysgenesis-associated AK2 protects hematopoietic stem and progenitor cell development from oxidative stress
- PMID: 26150473
- PMCID: PMC4516804
- DOI: 10.1084/jem.20141286
Reticular dysgenesis-associated AK2 protects hematopoietic stem and progenitor cell development from oxidative stress
Abstract
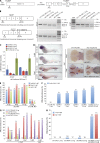
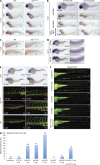
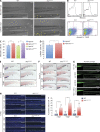
Adenylate kinases (AKs) are phosphotransferases that regulate the cellular adenine nucleotide composition and play a critical role in the energy homeostasis of all tissues. The AK2 isoenzyme is expressed in the mitochondrial intermembrane space and is mutated in reticular dysgenesis (RD), a rare form of severe combined immunodeficiency (SCID) in humans. RD is characterized by a maturation arrest in the myeloid and lymphoid lineages, leading to early onset, recurrent, and overwhelming infections. To gain insight into the pathophysiology of RD, we studied the effects of AK2 deficiency using the zebrafish model and induced pluripotent stem cells (iPSCs) derived from fibroblasts of an RD patient. In zebrafish, Ak2 deficiency affected hematopoietic stem and progenitor cell (HSPC) development with increased oxidative stress and apoptosis. AK2-deficient iPSCs recapitulated the characteristic myeloid maturation arrest at the promyelocyte stage and demonstrated an increased AMP/ADP ratio, indicative of an energy-depleted adenine nucleotide profile. Antioxidant treatment rescued the hematopoietic phenotypes in vivo in ak2 mutant zebrafish and restored differentiation of AK2-deficient iPSCs into mature granulocytes. Our results link hematopoietic cell fate in AK2 deficiency to cellular energy depletion and increased oxidative stress. This points to the potential use of antioxidants as a supportive therapeutic modality for patients with RD.
Figures






Comment in
-
Relieving oxidative stress in immune cells.J Exp Med. 2015 Jul 27;212(8):1140. doi: 10.1084/jem.2128insight1. J Exp Med. 2015. PMID: 26216601 Free PMC article. No abstract available.
References
-
- Antoine C., Müller S., Cant A., Cavazzana-Calvo M., Veys P., Vossen J., Fasth A., Heilmann C., Wulffraat N., Seger R., et al. . European Society for Immunodeficiency. 2003. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet. 361:553–560. 10.1016/S0140-6736(03)12513-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

