In vitro modeling of hyperpigmentation associated to neurofibromatosis type 1 using melanocytes derived from human embryonic stem cells
- PMID: 26150484
- PMCID: PMC4517235
- DOI: 10.1073/pnas.1501032112
In vitro modeling of hyperpigmentation associated to neurofibromatosis type 1 using melanocytes derived from human embryonic stem cells
Abstract
"Café-au-lait" macules (CALMs) and overall skin hyperpigmentation are early hallmarks of neurofibromatosis type 1 (NF1). One of the most frequent monogenic diseases, NF1 has subsequently been characterized with numerous benign Schwann cell-derived tumors. It is well established that neurofibromin, the NF1 gene product, is an antioncogene that down-regulates the RAS oncogene. In contrast, the molecular mechanisms associated with alteration of skin pigmentation have remained elusive. We have reassessed this issue by differentiating human embryonic stem cells into melanocytes. In the present study, we demonstrate that NF1 melanocytes reproduce the hyperpigmentation phenotype in vitro, and further characterize the link between loss of heterozygosity and the typical CALMs that appear over the general hyperpigmentation. Molecular mechanisms associated with these pathological phenotypes correlate with an increased activity of cAMP-mediated PKA and ERK1/2 signaling pathways, leading to overexpression of the transcription factor MITF and of the melanogenic enzymes tyrosinase and dopachrome tautomerase, all major players in melanogenesis. Finally, the hyperpigmentation phenotype can be rescued using specific inhibitors of these signaling pathways. These results open avenues for deciphering the pathological mechanisms involved in pigmentation diseases, and provide a robust assay for the development of new strategies for treating these diseases.
Keywords: disease modeling; embryonic stem cells; hyperpigmentation; melanocytes; neurofibromatosis type 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
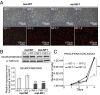






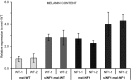



Comment in
-
What's up NF1?Pigment Cell Melanoma Res. 2016 Jan;29(1):4-5. doi: 10.1111/pcmr.12423. Epub 2015 Nov 3. Pigment Cell Melanoma Res. 2016. PMID: 26394792 No abstract available.
References
-
- Trovó-Marqui AB, Tajara EH. Neurofibromin: A general outlook. Clin Genet. 2006;70(1):1–13. - PubMed
-
- De Schepper S, Boucneau J, Lambert J, Messiaen L, Naeyaert J-M. Pigment cell-related manifestations in neurofibromatosis type 1: An overview. Pigment Cell Res. 2005;18(1):13–24. - PubMed
-
- Gutmann DH, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278(1):51–57. - PubMed
-
- Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplège A. Quality-of-life impairment in neurofibromatosis type 1: A cross-sectional study of 128 cases. Arch Dermatol. 2001;137(11):1421–1425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

