The EBF transcription factor Collier directly promotes Drosophila blood cell progenitor maintenance independently of the niche
- PMID: 26150488
- PMCID: PMC4517242
- DOI: 10.1073/pnas.1423967112
The EBF transcription factor Collier directly promotes Drosophila blood cell progenitor maintenance independently of the niche
Abstract
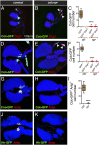
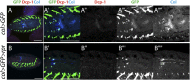
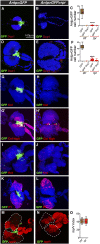
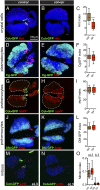
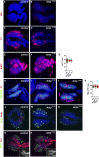
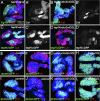
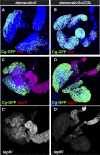
The maintenance of stem or progenitor cell fate relies on intrinsic factors as well as local cues from the cellular microenvironment and systemic signaling. In the lymph gland, an hematopoietic organ in Drosophila larva, a group of cells called the Posterior Signaling Centre (PSC), whose specification depends on the EBF transcription factor Collier (Col) and the HOX factor Antennapedia (Antp), has been proposed to form a niche required to maintain the pool of hematopoietic progenitors (prohemocytes). In contrast with this model, we show here that genetic ablation of the PSC does not cause an increase in blood cell differentiation or a loss of blood cell progenitors. Furthermore, although both col and Antp mutant larvae are devoid of PSC, the massive prohemocyte differentiation observed in col mutant is not phenocopied in Antp mutant. Interestingly, beside its expression in the PSC, Col is also expressed at low levels in prohemocytes and we show that this expression persists in PSC-ablated and Antp mutant larvae. Moreover, targeted knockdown and rescue experiments indicate that Col expression is required in the prohemocytes to prevent their differentiation. Together, our findings show that the PSC is dispensable for blood cell progenitor maintenance and reveal the key role of the conserved transcription factor Col as an intrinsic regulator of hematopoietic progenitor fate.
Keywords: Drosophila; EBF; hematopoiesis; stem cell niche.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

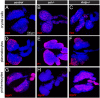
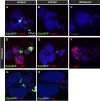
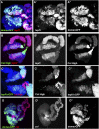
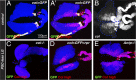
Comment in
-
Haematopoietic progenitor maintenance by EBF/Collier: beyond the Niche.Cell Cycle. 2015;14(22):3517-8. doi: 10.1080/15384101.2015.1093449. Cell Cycle. 2015. PMID: 26654595 Free PMC article. No abstract available.
Similar articles
-
Blood cell progenitor maintenance: Collier barks out of the niche.Fly (Austin). 2015;9(4):160-4. doi: 10.1080/19336934.2016.1151130. Fly (Austin). 2015. PMID: 26925971 Free PMC article.
-
Gene regulatory networks controlling hematopoietic progenitor niche cell production and differentiation in the Drosophila lymph gland.PLoS One. 2012;7(7):e41604. doi: 10.1371/journal.pone.0041604. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22911822 Free PMC article.
-
Two Independent Functions of Collier/Early B Cell Factor in the Control of Drosophila Blood Cell Homeostasis.PLoS One. 2016 Feb 11;11(2):e0148978. doi: 10.1371/journal.pone.0148978. eCollection 2016. PLoS One. 2016. PMID: 26866694 Free PMC article.
-
Drosophila as a Model to Study Cellular Communication Between the Hematopoietic Niche and Blood Progenitors Under Homeostatic Conditions and in Response to an Immune Stress.Front Immunol. 2021 Aug 16;12:719349. doi: 10.3389/fimmu.2021.719349. eCollection 2021. Front Immunol. 2021. PMID: 34484226 Free PMC article. Review.
-
The Posterior Signaling Center Is an Important Microenvironment for Homeostasis of the Drosophila Lymph Gland.Front Cell Dev Biol. 2020 May 21;8:382. doi: 10.3389/fcell.2020.00382. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32509789 Free PMC article. Review.
Cited by
-
Peeling Back the Layers of Lymph Gland Structure and Regulation.Int J Mol Sci. 2022 Jul 14;23(14):7767. doi: 10.3390/ijms23147767. Int J Mol Sci. 2022. PMID: 35887113 Free PMC article. Review.
-
Temporal specificity and heterogeneity of Drosophila immune cells.EMBO J. 2020 Jun 17;39(12):e104486. doi: 10.15252/embj.2020104486. Epub 2020 Mar 12. EMBO J. 2020. PMID: 32162708 Free PMC article.
-
Headcase is a Repressor of Lamellocyte Fate in Drosophila melanogaster.Genes (Basel). 2019 Mar 5;10(3):173. doi: 10.3390/genes10030173. Genes (Basel). 2019. PMID: 30841641 Free PMC article.
-
Advances in Myeloid-Like Cell Origins and Functions in the Model Organism Drosophila melanogaster.Microbiol Spectr. 2017 Jan;5(1):10.1128/microbiolspec.mchd-0038-2016. doi: 10.1128/microbiolspec.MCHD-0038-2016. Microbiol Spectr. 2017. PMID: 28102122 Free PMC article. Review.
-
The matrix glycoprotein Papilin maintains the haematopoietic progenitor pool in Drosophila lymph glands.Development. 2025 Apr 1;152(7):dev204367. doi: 10.1242/dev.204367. Epub 2025 Apr 10. Development. 2025. PMID: 40094323 Free PMC article.
References
-
- Warr MR, Pietras EM, Passegué E. Mechanisms controlling hematopoietic stem cell functions during normal hematopoiesis and hematological malignancies. Wiley Interdiscip Rev Syst Biol Med. 2011;3(6):681–701. - PubMed
-
- Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36(9):1019–1023. - PubMed
-
- Krzemien J, Crozatier M, Vincent A. Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int J Dev Biol. 2010;54(6-7):1117–1125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

