Chloroplasts extend stromules independently and in response to internal redox signals
- PMID: 26150490
- PMCID: PMC4538653
- DOI: 10.1073/pnas.1511570112
Chloroplasts extend stromules independently and in response to internal redox signals
Abstract
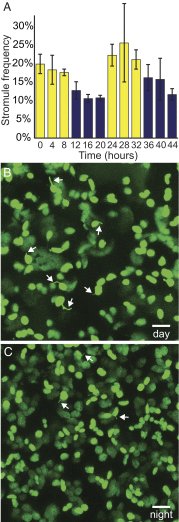
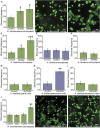
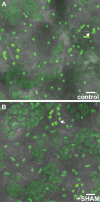
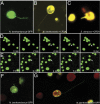
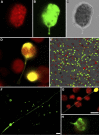
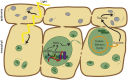
A fundamental mystery of plant cell biology is the occurrence of "stromules," stroma-filled tubular extensions from plastids (such as chloroplasts) that are universally observed in plants but whose functions are, in effect, completely unknown. One prevalent hypothesis is that stromules exchange signals or metabolites between plastids and other subcellular compartments, and that stromules are induced during stress. Until now, no signaling mechanisms originating within the plastid have been identified that regulate stromule activity, a critical missing link in this hypothesis. Using confocal and superresolution 3D microscopy, we have shown that stromules form in response to light-sensitive redox signals within the chloroplast. Stromule frequency increased during the day or after treatment with chemicals that produce reactive oxygen species specifically in the chloroplast. Silencing expression of the chloroplast NADPH-dependent thioredoxin reductase, a central hub in chloroplast redox signaling pathways, increased chloroplast stromule frequency, whereas silencing expression of nuclear genes related to plastid genome expression and tetrapyrrole biosynthesis had no impact on stromules. Leucoplasts, which are not photosynthetic, also made more stromules in the daytime. Leucoplasts did not respond to the same redox signaling pathway but instead increased stromule formation when exposed to sucrose, a major product of photosynthesis, although sucrose has no impact on chloroplast stromule frequency. Thus, different types of plastids make stromules in response to distinct signals. Finally, isolated chloroplasts could make stromules independently after extraction from the cytoplasm, suggesting that chloroplast-associated factors are sufficient to generate stromules. These discoveries demonstrate that chloroplasts are remarkably autonomous organelles that alter their stromule frequency in reaction to internal signal transduction pathways.
Keywords: chloroplasts; leucoplasts; light signaling; redox signaling; stromules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Comment in
-
Reactive oxygen species signal chloroplasts to extend themselves.Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):9799-800. doi: 10.1073/pnas.1512645112. Epub 2015 Jul 23. Proc Natl Acad Sci U S A. 2015. PMID: 26204917 Free PMC article. No abstract available.
References
-
- Koussevitzky S, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316(5825):715–719. - PubMed
-
- Xiao Y, et al. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149(7):1525–1535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

