Optimized deep-targeted proteotranscriptomic profiling reveals unexplored Conus toxin diversity and novel cysteine frameworks
- PMID: 26150494
- PMCID: PMC4517256
- DOI: 10.1073/pnas.1501334112
Optimized deep-targeted proteotranscriptomic profiling reveals unexplored Conus toxin diversity and novel cysteine frameworks
Erratum in
-
Correction for Lavergne et al., Optimized deep-targeted proteotranscriptomic profiling reveals unexplored Conus toxin diversity and novel cysteine frameworks.Proc Natl Acad Sci U S A. 2015 Nov 10;112(45):E6253. doi: 10.1073/pnas.1520243112. Epub 2015 Oct 26. Proc Natl Acad Sci U S A. 2015. PMID: 26504245 Free PMC article. No abstract available.
Abstract
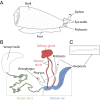
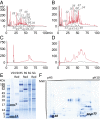
Cone snails are predatory marine gastropods characterized by a sophisticated venom apparatus responsible for the biosynthesis and delivery of complex mixtures of cysteine-rich toxin peptides. These conotoxins fold into small highly structured frameworks, allowing them to potently and selectively interact with heterologous ion channels and receptors. Approximately 2,000 toxins from an estimated number of >70,000 bioactive peptides have been identified in the genus Conus to date. Here, we describe a high-resolution interrogation of the transcriptomes (available at www.ddbj.nig.ac.jp) and proteomes of the diverse compartments of the Conus episcopatus venom apparatus. Using biochemical and bioinformatic tools, we found the highest number of conopeptides yet discovered in a single Conus specimen, with 3,305 novel precursor toxin sequences classified into 9 known superfamilies (A, I1, I2, M, O1, O2, S, T, Z), and identified 16 new superfamilies showing unique signal peptide signatures. We were also able to depict the largest population of venom peptides containing the pharmacologically active C-C-CC-C-C inhibitor cystine knot and CC-C-C motifs (168 and 44 toxins, respectively), as well as 208 new conotoxins displaying odd numbers of cysteine residues derived from known conotoxin motifs. Importantly, six novel cysteine-rich frameworks were revealed which may have novel pharmacology. Finally, analyses of codon usage bias and RNA-editing processes of the conotoxin transcripts demonstrate a specific conservation of the cysteine skeleton at the nucleic acid level and provide new insights about the origin of sequence hypervariablity in mature toxin regions.
Keywords: bioinformatic; conotoxin; cysteine-rich peptides; proteomic; transcriptomic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Bouchet P, Gofas S. 2014. Conus Linnaeus, 1758. World Register of Marine Species. Available at www.marinespecies.org. Accessed April 29, 2015.
-
- Duda TF, Jr, Kohn AJ. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Mol Phylogenet Evol. 2005;34(2):257–272. - PubMed
-
- Freeman SE, Turner RJ, Silva SR. The venom and venom apparatus of the marine gastropod Conus striatus Linne. Toxicon. 1974;12(6):587–592. - PubMed
-
- Spengler HA, Kohn AJ. Comparative external morphology of the Conus osphradium (Mollusca: Gastropoda) J Zool. 1995;235(3):439–453.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

