Structure of a herpesvirus nuclear egress complex subunit reveals an interaction groove that is essential for viral replication
- PMID: 26150520
- PMCID: PMC4517201
- DOI: 10.1073/pnas.1511140112
Structure of a herpesvirus nuclear egress complex subunit reveals an interaction groove that is essential for viral replication
Abstract
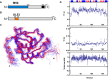
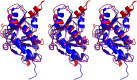
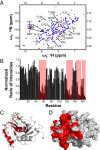
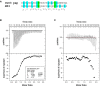
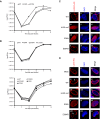
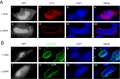
Herpesviruses require a nuclear egress complex (NEC) for efficient transit of nucleocapsids from the nucleus to the cytoplasm. The NEC orchestrates multiple steps during herpesvirus nuclear egress, including disruption of nuclear lamina and particle budding through the inner nuclear membrane. In the important human pathogen human cytomegalovirus (HCMV), this complex consists of nuclear membrane protein UL50, and nucleoplasmic protein UL53, which is recruited to the nuclear membrane through its interaction with UL50. Here, we present an NMR-determined solution-state structure of the murine CMV homolog of UL50 (M50; residues 1-168) with a strikingly intricate protein fold that is matched by no other known protein folds in its entirety. Using NMR methods, we mapped the interaction of M50 with a highly conserved UL53-derived peptide, corresponding to a segment that is required for heterodimerization. The UL53 peptide binding site mapped onto an M50 surface groove, which harbors a large cavity. Point mutations of UL50 residues corresponding to surface residues in the characterized M50 heterodimerization interface substantially decreased UL50-UL53 binding in vitro, eliminated UL50-UL53 colocalization, prevented disruption of nuclear lamina, and halted productive virus replication in HCMV-infected cells. Our results provide detailed structural information on a key protein-protein interaction involved in nuclear egress and suggest that NEC subunit interactions can be an attractive drug target.
Keywords: NMR; cytomegalovirus; drug target; herpesvirus; protein–protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Human cytomegalovirus UL50 and UL53 recruit viral protein kinase UL97, not protein kinase C, for disruption of nuclear lamina and nuclear egress in infected cells.J Virol. 2014 Jan;88(1):249-62. doi: 10.1128/JVI.02358-13. Epub 2013 Oct 23. J Virol. 2014. PMID: 24155370 Free PMC article.
-
Biochemical, biophysical, and mutational analyses of subunit interactions of the human cytomegalovirus nuclear egress complex.J Virol. 2009 Apr;83(7):2996-3006. doi: 10.1128/JVI.02441-08. Epub 2009 Jan 19. J Virol. 2009. PMID: 19153235 Free PMC article.
-
Unexpected features and mechanism of heterodimer formation of a herpesvirus nuclear egress complex.EMBO J. 2015 Dec 2;34(23):2937-52. doi: 10.15252/embj.201592651. Epub 2015 Oct 28. EMBO J. 2015. PMID: 26511021 Free PMC article.
-
Nuclear Egress Complexes of HCMV and Other Herpesviruses: Solving the Puzzle of Sequence Coevolution, Conserved Structures and Subfamily-Spanning Binding Properties.Viruses. 2020 Jun 24;12(6):683. doi: 10.3390/v12060683. Viruses. 2020. PMID: 32599939 Free PMC article. Review.
-
'Getting Better'-Is It a Feasible Strategy of Broad Pan-Antiherpesviral Drug Targeting by Using the Nuclear Egress-Directed Mechanism?Int J Mol Sci. 2024 Feb 29;25(5):2823. doi: 10.3390/ijms25052823. Int J Mol Sci. 2024. PMID: 38474070 Free PMC article. Review.
Cited by
-
Host and Viral Factors Involved in Nuclear Egress of Herpes Simplex Virus 1.Viruses. 2021 Apr 25;13(5):754. doi: 10.3390/v13050754. Viruses. 2021. PMID: 33923040 Free PMC article. Review.
-
A small molecule exerts selective antiviral activity by targeting the human cytomegalovirus nuclear egress complex.PLoS Pathog. 2023 Nov 17;19(11):e1011781. doi: 10.1371/journal.ppat.1011781. eCollection 2023 Nov. PLoS Pathog. 2023. PMID: 37976321 Free PMC article.
-
Human Cytomegalovirus Nuclear Egress Complex Subunit, UL53, Associates with Capsids and Myosin Va, but Is Not Important for Capsid Localization towards the Nuclear Periphery.Viruses. 2022 Feb 26;14(3):479. doi: 10.3390/v14030479. Viruses. 2022. PMID: 35336886 Free PMC article.
-
A Role for Myosin Va in Human Cytomegalovirus Nuclear Egress.J Virol. 2018 Feb 26;92(6):e01849-17. doi: 10.1128/JVI.01849-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298889 Free PMC article.
-
Mechanism of Nuclear Lamina Disruption and the Role of pUS3 in HSV-1 Nuclear Egress.J Virol. 2021 Apr 26;95(10):e02432-20. doi: 10.1128/JVI.02432-20. Epub 2021 Mar 3. J Virol. 2021. PMID: 33658339 Free PMC article.
References
-
- Pellett PE, Roizman B. Herpesviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 6th Ed. Vol 2. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia: 2013. pp. 1802–1822.
-
- National Center for Immunization and Respiratory Diseases Division of Viral Diseases (2010) CDC: Cytomegalovirus (CMV) and Congenital CMV Infection: Overview (Centers for Disease Control, Atlanta, GA)
-
- Boppana SB, Fowler KB. Persistence in the population: Epidemiology and transmisson. In: Arvin AM, Campadelli-Fiume G, Mocarski E, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge Univ Press; Cambridge, United Kingdom: 2007. pp. 795–813.
-
- Mettenleiter TC. Budding events in herpesvirus morphogenesis. Virus Res. 2004;106(2):167–180. - PubMed
-
- Mettenleiter TC, Müller F, Granzow H, Klupp BG. The way out: What we know and do not know about herpesvirus nuclear egress. Cell Microbiol. 2013;15(2):170–178. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

