Skip residues modulate the structural properties of the myosin rod and guide thick filament assembly
- PMID: 26150528
- PMCID: PMC4517226
- DOI: 10.1073/pnas.1505813112
Skip residues modulate the structural properties of the myosin rod and guide thick filament assembly
Abstract
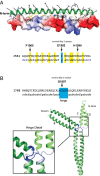
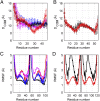
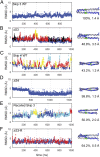
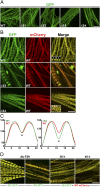
The rod of sarcomeric myosins directs thick filament assembly and is characterized by the insertion of four skip residues that introduce discontinuities in the coiled-coil heptad repeats. We report here that the regions surrounding the first three skip residues share high structural similarity despite their low sequence homology. Near each of these skip residues, the coiled-coil transitions to a nonclose-packed structure inducing local relaxation of the superhelical pitch. Moreover, molecular dynamics suggest that these distorted regions can assume different conformationally stable states. In contrast, the last skip residue region constitutes a true molecular hinge, providing C-terminal rod flexibility. Assembly of myosin with mutated skip residues in cardiomyocytes shows that the functional importance of each skip residue is associated with rod position and reveals the unique role of the molecular hinge in promoting myosin antiparallel packing. By defining the biophysical properties of the rod, the structures and molecular dynamic calculations presented here provide insight into thick filament formation, and highlight the structural differences occurring between the coiled-coils of myosin and the stereotypical tropomyosin. In addition to extending our knowledge into the conformational and biological properties of coiled-coil discontinuities, the molecular characterization of the four myosin skip residues also provides a guide to modeling the effects of rod mutations causing cardiac and skeletal myopathies.
Keywords: cardiac/skeletal myopathies; coiled-coils; molecular dynamics; myosin; protein structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A1603P and K1617del, Mutations in β-Cardiac Myosin Heavy Chain that Cause Laing Early-Onset Distal Myopathy, Affect Secondary Structure and Filament Formation In Vitro and In Vivo.J Mol Biol. 2018 May 11;430(10):1459-1478. doi: 10.1016/j.jmb.2018.04.006. Epub 2018 Apr 14. J Mol Biol. 2018. PMID: 29660325 Free PMC article.
-
Mutations in Drosophila myosin rod cause defects in myofibril assembly.J Mol Biol. 2012 May 25;419(1-2):22-40. doi: 10.1016/j.jmb.2012.02.025. Epub 2012 Feb 24. J Mol Biol. 2012. PMID: 22370558
-
A 29 residue region of the sarcomeric myosin rod is necessary for filament formation.J Mol Biol. 1997 Feb 21;266(2):317-30. doi: 10.1006/jmbi.1996.0790. J Mol Biol. 1997. PMID: 9047366
-
Structural implications of β-cardiac myosin heavy chain mutations in human disease.Anat Rec (Hoboken). 2014 Sep;297(9):1670-80. doi: 10.1002/ar.22973. Anat Rec (Hoboken). 2014. PMID: 25125180 Review.
-
Determining structure/function relationships for sarcomeric myosin heavy chain by genetic and transgenic manipulation of Drosophila.Microsc Res Tech. 2000 Sep 15;50(6):430-42. doi: 10.1002/1097-0029(20000915)50:6<430::AID-JEMT2>3.0.CO;2-E. Microsc Res Tech. 2000. PMID: 10998634 Review.
Cited by
-
Electrostatic and bending energies predict staggering and splaying in nonmuscle myosin II minifilaments.PLoS Comput Biol. 2020 Jul 6;16(7):e1007801. doi: 10.1371/journal.pcbi.1007801. eCollection 2020 Jul. PLoS Comput Biol. 2020. PMID: 32628657 Free PMC article.
-
Structure of myosin filaments from relaxed Lethocerus flight muscle by cryo-EM at 6 Å resolution.Sci Adv. 2016 Sep 30;2(9):e1600058. doi: 10.1126/sciadv.1600058. eCollection 2016 Sep. Sci Adv. 2016. PMID: 27704041 Free PMC article.
-
The small molecule chemical compound cinobufotalin attenuates resistance to DDP by inducing ENKUR expression to suppress MYH9-mediated c-Myc deubiquitination in lung adenocarcinoma.Acta Pharmacol Sin. 2022 Oct;43(10):2687-2695. doi: 10.1038/s41401-022-00890-x. Epub 2022 Mar 16. Acta Pharmacol Sin. 2022. PMID: 35296779 Free PMC article.
-
Human skeletal myopathy myosin mutations disrupt myosin head sequestration.JCI Insight. 2023 Nov 8;8(21):e172322. doi: 10.1172/jci.insight.172322. JCI Insight. 2023. PMID: 37788100 Free PMC article.
-
The myosin II coiled-coil domain atomic structure in its native environment.Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2024151118. doi: 10.1073/pnas.2024151118. Proc Natl Acad Sci U S A. 2021. PMID: 33782130 Free PMC article.
References
-
- Craig R. Structure of A-segments from frog and rabbit skeletal muscle. J Mol Biol. 1977;109(1):69–81. - PubMed
-
- Gautel M. The sarcomeric cytoskeleton: Who picks up the strain? Curr Opin Cell Biol. 2011;23(1):39–46. - PubMed
-
- Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16(2):204–212. - PubMed
-
- McLachlan AD, Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982;299(5880):226–231. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

