The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis
- PMID: 26150532
- PMCID: PMC4530049
- DOI: 10.4049/jimmunol.1500496
The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis
Abstract
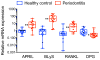
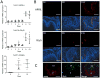
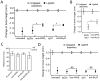
B-lineage cells (B lymphocytes and plasma cells) predominate in the inflammatory infiltrate of human chronic periodontitis. However, their role in disease pathogenesis and the factors responsible for their persistence in chronic lesions are poorly understood. In this regard, two cytokines of the TNF ligand superfamily, a proliferation-inducing ligand (APRIL) and B-lymphocyte stimulator (BLyS), are important for the survival, proliferation, and maturation of B cells. Thus, we hypothesized that APRIL and/or BLyS are upregulated in periodontitis and contribute to induction of periodontal bone loss. This hypothesis was addressed in both human and mouse experimental systems. We show that, relative to healthy controls, the expression of APRIL and BLyS mRNA and protein was upregulated in natural and experimental periodontitis in humans and mice, respectively. The elevated expression of these cytokines correlated with increased numbers of B cells/plasma cells in both species. Moreover, APRIL and BLyS partially colocalized with κ L chain-expressing B-lineage cells at the epithelial-connective tissue interface. Ligature-induced periodontitis resulted in significantly less bone loss in B cell-deficient mice compared with wild-type controls. Ab-mediated neutralization of APRIL or BLyS diminished the number of B cells in the gingival tissue and inhibited bone loss in wild-type, but not in B cell-deficient, mice. In conclusion, B cells and specific cytokines involved in their growth and differentiation contribute to periodontal bone loss. Moreover, APRIL and BLyS have been identified as potential therapeutic targets in periodontitis.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures






Comment in
-
Comment on "The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis.".J Immunol. 2015 Dec 1;195(11):5099. doi: 10.4049/jimmunol.1502059. J Immunol. 2015. PMID: 26589743 No abstract available.
-
Response to Comment on "The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis".J Immunol. 2015 Dec 1;195(11):5099-100. doi: 10.4049/jimmunol.1502066. J Immunol. 2015. PMID: 26589744 No abstract available.
References
-
- Garlet GP. Destructive and protective roles of cytokines in periodontitis: A reappraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. - PubMed
-
- Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE015254/DE/NIDCR NIH HHS/United States
- AI068730/AI/NIAID NIH HHS/United States
- DE021685/DE/NIDCR NIH HHS/United States
- DE017138/DE/NIDCR NIH HHS/United States
- R01 AI054488/AI/NIAID NIH HHS/United States
- DE015254/DE/NIDCR NIH HHS/United States
- R01 DE023836/DE/NIDCR NIH HHS/United States
- R01 DE024160/DE/NIDCR NIH HHS/United States
- R01 DE021685/DE/NIDCR NIH HHS/United States
- DE024716/DE/NIDCR NIH HHS/United States
- DE023836/DE/NIDCR NIH HHS/United States
- R01 DE017138/DE/NIDCR NIH HHS/United States
- P01 AI068730/AI/NIAID NIH HHS/United States
- R01 DE024716/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

