Parasite Proximity Drives the Expansion of Regulatory T Cells in Peyer's Patches following Intestinal Helminth Infection
- PMID: 26150538
- PMCID: PMC4534664
- DOI: 10.1128/IAI.00266-15
Parasite Proximity Drives the Expansion of Regulatory T Cells in Peyer's Patches following Intestinal Helminth Infection
Abstract
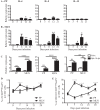
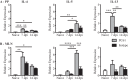
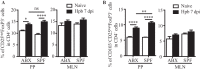
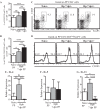
Helminth infections are typically chronic in nature; however, the exact molecular mechanisms by which these parasites promote or thwart host immunity remain unclear. Worm expulsion requires the differentiation of CD4(+) T cells into Th2 cells, while regulatory T cells (Tregs) act to dampen the extent of the Th2 response. Priming of T cells requires drainage or capture of antigens within lymphoid tissues, and in the case of intestinal helminths, such sites include the mucosa-associated Peyer's patches (PPs) and the draining mesenteric lymph nodes (MLN). To gain insight into when and where the activation of the adaptive T cell response takes place following intestinal helminth infection, we analyzed Th2 and Treg responses in the PPs and MLN following infection with the murine intestinal helminth Heligmosomoides polygyrus bakeri. Protective Th2 responses were observed to be largely restricted to the MLN, while a greater expansion of Tregs occurred within the PPs. Interestingly, those PPs that formed a contact with the parasite showed the greatest degree of Treg expansion and no evidence of type 2 cytokine production, indicating that the parasite may secrete products that act in a local manner to selectively promote Treg expansion. This view was supported by the finding that H. polygyrus bakeri larvae could promote Treg proliferation in vitro. Taken together, these data indicate that different degrees of Treg expansion and type 2 cytokine production occur within the PPs and MLN following infection with the intestinal helminth H. polygyrus bakeri and indicate that these organs exhibit differential responses following infection with intestinal helminths.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3.J Immunol. 1993 Apr 15;150(8 Pt 1):3434-41. J Immunol. 1993. PMID: 8468481
-
Heligmosomoides polygyrus bakeri infection activates colonic Foxp3+ T cells enhancing their capacity to prevent colitis.J Immunol. 2013 Aug 15;191(4):1927-34. doi: 10.4049/jimmunol.1201457. Epub 2013 Jul 12. J Immunol. 2013. PMID: 23851695 Free PMC article.
-
Eosinophils are required to suppress Th2 responses in Peyer's patches during intestinal infection by nematodes.Mucosal Immunol. 2017 May;10(3):661-672. doi: 10.1038/mi.2016.93. Epub 2016 Nov 2. Mucosal Immunol. 2017. PMID: 27805618
-
MicroRNA-mediated regulation of immune responses to intestinal helminth infections.Parasite Immunol. 2017 Feb;39(2). doi: 10.1111/pim.12406. Parasite Immunol. 2017. PMID: 27977850 Review.
-
Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus.Semin Immunopathol. 2012 Nov;34(6):829-46. doi: 10.1007/s00281-012-0347-3. Epub 2012 Oct 11. Semin Immunopathol. 2012. PMID: 23053394 Free PMC article. Review.
Cited by
-
Heligmosomoides bakeri and Toxoplasma gondii co-infection leads to increased mortality associated with changes in immune resistance in the lymphoid compartment and disease pathology.PLoS One. 2024 Jul 1;19(7):e0292408. doi: 10.1371/journal.pone.0292408. eCollection 2024. PLoS One. 2024. PMID: 38950025 Free PMC article.
-
CD1d-Dependent iNKT Cells Control DSS-Induced Colitis in a Mouse Model of IFNγ-Mediated Hyperinflammation by Increasing IL22-Secreting ILC3 Cells.Int J Mol Sci. 2021 Jan 27;22(3):1250. doi: 10.3390/ijms22031250. Int J Mol Sci. 2021. PMID: 33513946 Free PMC article.
-
Oral delivery of a functional algal-expressed TGF-β mimic halts colitis in a murine DSS model.J Biotechnol. 2021 Nov 10;340:1-12. doi: 10.1016/j.jbiotec.2021.08.006. Epub 2021 Aug 12. J Biotechnol. 2021. PMID: 34390759 Free PMC article.
-
Primary Heligmosomoides polygyrus bakeri infection induces myeloid-derived suppressor cells that suppress CD4+ Th2 responses and promote chronic infection.Mucosal Immunol. 2017 Jan;10(1):238-249. doi: 10.1038/mi.2016.36. Epub 2016 Apr 13. Mucosal Immunol. 2017. PMID: 27072608
-
The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation.Immunity. 2015 Nov 17;43(5):998-1010. doi: 10.1016/j.immuni.2015.09.012. Epub 2015 Oct 27. Immunity. 2015. PMID: 26522986 Free PMC article.
References
-
- Krolewiecki A, Lammie P, Jacobson J, Gabrielli A-F, Levecke B, Socias E, Arias L, Sosa N, Abraham D, Cimino R, Echazú A, Crudo F, Vercruysse J, Albonico M. 2013. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis 7:e2165. doi:10.1371/journal.pntd.0002165. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

