Heparan Sulfate Modulates Neutrophil and Endothelial Function in Antibacterial Innate Immunity
- PMID: 26150541
- PMCID: PMC4534644
- DOI: 10.1128/IAI.00545-15
Heparan Sulfate Modulates Neutrophil and Endothelial Function in Antibacterial Innate Immunity
Abstract
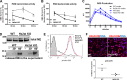
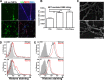
Recently, we showed that endothelial heparan sulfate facilitates entry of a bacterial pathogen into the central nervous system. Here, we show that normal bactericidal activity of neutrophils is influenced by the sulfation pattern of heparan sulfate. Inactivation of heparan sulfate uronyl 2-O-sulfotransferase (Hs2st) in neutrophils substantially reduced their bactericidal activity, and Hs2st deficiency rendered mice more susceptible to systemic infection with the pathogenic bacterium group B Streptococcus. Specifically, altered sulfation of heparan sulfate in mutant neutrophils affected formation of neutrophil extracellular traps while not influencing phagocytosis, production of reactive oxygen species, or secretion of granular proteases. Heparan sulfate proteoglycan(s) is present in neutrophil extracellular traps, modulates histone affinity, and modulates their microbial activity. Hs2st-deficient brain endothelial cells show enhanced binding to group B Streptococcus and are more susceptible to apoptosis, likely contributing to the observed increase in dissemination of group B Streptococcus into the brain of Hs2st-deficient mice following intravenous challenge. Taken together, our data provide strong evidence that heparan sulfate from both neutrophils and the endothelium plays important roles in modulating innate immunity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

