Cytoplasmic LSM-1 protein regulates stress responses through the insulin/IGF-1 signaling pathway in Caenorhabditis elegans
- PMID: 26150554
- PMCID: PMC4536316
- DOI: 10.1261/rna.052324.115
Cytoplasmic LSM-1 protein regulates stress responses through the insulin/IGF-1 signaling pathway in Caenorhabditis elegans
Abstract
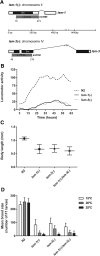
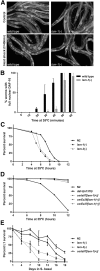
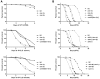
Genes coding for members of the Sm-like (LSm) protein family are conserved through evolution from prokaryotes to humans. These proteins have been described as forming homo- or heterocomplexes implicated in a broad range of RNA-related functions. To date, the nuclear LSm2-8 and the cytoplasmic LSm1-7 heteroheptamers are the best characterized complexes in eukaryotes. Through a comprehensive functional study of the LSm family members, we found that lsm-1 and lsm-3 are not essential for C. elegans viability, but their perturbation, by RNAi or mutations, produces defects in development, reproduction, and motility. We further investigated the function of lsm-1, which encodes the distinctive protein of the cytoplasmic complex. RNA-seq analysis of lsm-1 mutants suggests that they have impaired Insulin/IGF-1 signaling (IIS), which is conserved in metazoans and involved in the response to various types of stress through the action of the FOXO transcription factor DAF-16. Further analysis using a DAF-16::GFP reporter indicated that heat stress-induced translocation of DAF-16 to the nuclei is dependent on lsm-1. Consistent with this, we observed that lsm-1 mutants display heightened sensitivity to thermal stress and starvation, while overexpression of lsm-1 has the opposite effect. We also observed that under stress, cytoplasmic LSm proteins aggregate into granules in an LSM-1-dependent manner. Moreover, we found that lsm-1 and lsm-3 are required for other processes regulated by the IIS pathway, such as aging and pathogen resistance.
Keywords: Caenorhabditis elegans; LSM, daf-16; P bodies; stress granules; stress response.
© 2015 Cornes et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures

References
-
- Buchan JR, Nissan T, Parker R. 2010. Analyzing P-bodies and stress granules in Saccharomyces cerevisiae. Methods Enzymol 470: 619–640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
