A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy
- PMID: 26150601
- PMCID: PMC5264222
- DOI: 10.1136/annrheumdis-2015-207588
A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy
Abstract
Objectives: To compare the efficacy and safety of SB4 (an etanercept biosimilar) with reference product etanercept (ETN) in patients with moderate to severe rheumatoid arthritis (RA) despite methotrexate (MTX) therapy.
Methods: This is a phase III, randomised, double-blind, parallel-group, multicentre study with a 24-week primary endpoint. Patients with moderate to severe RA despite MTX treatment were randomised to receive weekly dose of 50 mg of subcutaneous SB4 or ETN. The primary endpoint was the American College of Rheumatology 20% (ACR20) response at week 24. Other efficacy endpoints as well as safety, immunogenicity and pharmacokinetic parameters were also measured.
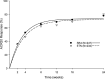
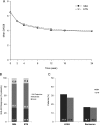
Results: 596 patients were randomised to either SB4 (N=299) or ETN (N=297). The ACR20 response rate at week 24 in the per-protocol set was 78.1% for SB4 and 80.3% for ETN. The 95% CI of the adjusted treatment difference was -9.41% to 4.98%, which is completely contained within the predefined equivalence margin of -15% to 15%, indicating therapeutic equivalence between SB4 and ETN. Other efficacy endpoints and pharmacokinetic endpoints were comparable. The incidence of treatment-emergent adverse events was comparable (55.2% vs 58.2%), and the incidence of antidrug antibody development up to week 24 was lower in SB4 compared with ETN (0.7% vs 13.1%).
Conclusions: SB4 was shown to be equivalent with ETN in terms of efficacy at week 24. SB4 was well tolerated with a lower immunogenicity profile. The safety profile of SB4 was comparable with that of ETN.
Trial registration numbers: NCT01895309, EudraCT 2012-005026-30.
Keywords: Anti-TNF; DMARDs (biologic); Rheumatoid Arthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
All authors received funding for clinical research from Samsung Bioepis: PE received consulting fees; JV, AS, PL, WP, AB, VT, VMZ, BS, RM, and AABR received research grants; SYC and JG are full-time employee of Samsung Bioepis. In addition, PE reports receiving grant/research support from AbbVie, Pfizer and consultancy fee from AbbVie, BMS, Pfizer, USB, MSD, Roche, Novartis, Takeda, Lilly; JV served on speakers bureau for USB, Pfizer, AbbVie, MSD; PL reports receiving grant/research support from Roche, MSD, Janssen, Novo-Nordisk, UCB, Pfizer, Novartis, GSK, BM, served as paid instructor for Novo-Nordisk, and served on speakers bureau for MSD, UCB, Roche, Amgen; AB reports receiving grant/research support from AbbVie.
Figures


Comment in
-
Comparing the immunogenicity of the etanercept biosimilar SB4 with the innovator etanercept: another consideration.Ann Rheum Dis. 2016 Jul;75(7):e37. doi: 10.1136/annrheumdis-2016-209502. Epub 2016 Mar 31. Ann Rheum Dis. 2016. PMID: 27034452 No abstract available.
-
Anti Etanercept and anti SB4 antibodies detection: impact of the assay method.Ann Rheum Dis. 2016 Jul;75(7):e39. doi: 10.1136/annrheumdis-2016-209665. Epub 2016 Apr 29. Ann Rheum Dis. 2016. PMID: 27130906 No abstract available.
-
Response to: 'Comparing the immunogenicity of the etanercept biosimilar SB4 with the innovator etanercept: another consideration' by Marshall et al.Ann Rheum Dis. 2016 Jul;75(7):e38. doi: 10.1136/annrheumdis-2016-209517. Epub 2016 May 4. Ann Rheum Dis. 2016. PMID: 27147708 No abstract available.
-
Confirmation on the immunogenicity assay used in the SB4 phase III study: response to the comments by Meacci et al.Ann Rheum Dis. 2016 Jul;75(7):e40. doi: 10.1136/annrheumdis-2016-209696. Epub 2016 May 17. Ann Rheum Dis. 2016. PMID: 27190097 No abstract available.
-
'Lower anti-drug antibodies with etanercept biosimilar: can Ctrough explain the differences?'.Ann Rheum Dis. 2016 Sep;75(9):e60. doi: 10.1136/annrheumdis-2016-210089. Epub 2016 Jul 7. Ann Rheum Dis. 2016. PMID: 27390363 No abstract available.
-
Difference between Enbrel and Benepali treatment groups in 'hepatobiliary disorders'.Ann Rheum Dis. 2016 Oct;75(10):e64. doi: 10.1136/annrheumdis-2016-210101. Epub 2016 Jul 18. Ann Rheum Dis. 2016. PMID: 27432354 No abstract available.
-
Response to: 'Lower anti-drug antibodies with etanercept biosimilar: Can Ctrough explain the differences' by Shah.Ann Rheum Dis. 2016 Sep;75(9):e61. doi: 10.1136/annrheumdis-2016-210114. Epub 2016 Jul 19. Ann Rheum Dis. 2016. PMID: 27435063 No abstract available.
-
Difference between SB4 and reference etanercept in the hepatobiliary disorders not considered to be caused by SB4: response to letter by Scheinberg and Azevedo.Ann Rheum Dis. 2016 Oct;75(10):e65. doi: 10.1136/annrheumdis-2016-210127. Epub 2016 Aug 8. Ann Rheum Dis. 2016. PMID: 27502892 No abstract available.
-
Clinical trials of biosimilars should become more similar.Ann Rheum Dis. 2017 Jan;76(1):4-6. doi: 10.1136/annrheumdis-2015-208113. Epub 2016 Aug 25. Ann Rheum Dis. 2017. PMID: 27566795 No abstract available.
References
-
- Murray KM, Dahl SL. Recombinant human tumor necrosis factor receptor (p75) Fc fusion protein (TNFR:Fc) in rheumatoid arthritis. Ann Pharmacother 1997;31:1335–8. - PubMed
-
- Emery P, Breedveld FC, Hall S, et al. . Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. 10.1016/S0140-6736(08)61000-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

