The role of maternal obesity in the risk of neuropsychiatric disorders
- PMID: 26150767
- PMCID: PMC4471351
- DOI: 10.3389/fnins.2015.00194
The role of maternal obesity in the risk of neuropsychiatric disorders
Abstract
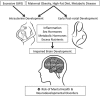
Recent evidence indicates that perinatal exposure to maternal obesity, metabolic disease, including diabetes and hypertension, and unhealthy maternal diet has a long-term impact on offspring behavior and physiology. During the past three decades, the prevalence of both obesity and neuropsychiatric disorders has rapidly increased. Epidemiologic studies provide evidence that maternal obesity and metabolic complications increase the risk of attention deficit hyperactivity disorder (ADHD), autism spectrum disorders, anxiety, depression, schizophrenia, eating disorders (food addiction, anorexia nervosa, and bulimia nervosa), and impairments in cognition in offspring. Animal models of maternal high-fat diet (HFD) induced obesity also document persistent changes in offspring behavior and impairments in critical neural circuitry. Animals exposed to maternal obesity and HFD consumption display hyperactivity, impairments in social behavior, increased anxiety-like and depressive-like behaviors, substance addiction, food addiction, and diminished cognition. During development, these offspring are exposed to elevated levels of nutrients (fatty acids, glucose), hormones (leptin, insulin), and inflammatory factors (C-reactive protein, interleukin, and tumor necrosis factor). Such factors appear to permanently change neuroendocrine regulation and brain development in offspring. In addition, inflammation of the offspring brain during gestation impairs the development of neural pathways critical in the regulation of behavior, such as serotoninergic, dopaminergic, and melanocortinergic systems. Dysregulation of these circuits increases the risk of mental health disorders. Given the high rates of obesity in most developed nations, it is critical that the mechanisms by which maternal obesity programs offspring behavior are thoroughly characterized. Such knowledge will be critical in the development of preventative strategies and therapeutic interventions.
Keywords: attention deficit hyperactivity disorder; autism spectrum disorders; eating disorders; metabolic programming; mood disorders; schizophrenia.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

