Oxidized cholesterol as the driving force behind the development of Alzheimer's disease
- PMID: 26150787
- PMCID: PMC4473000
- DOI: 10.3389/fnagi.2015.00119
Oxidized cholesterol as the driving force behind the development of Alzheimer's disease
Abstract
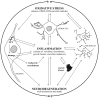
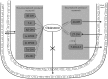
Alzheimer's disease (AD), the most common neurodegenerative disorder associated with dementia, is typified by the pathological accumulation of amyloid Aβ peptides and neurofibrillary tangles (NFT) within the brain. Considerable evidence indicates that many events contribute to AD progression, including oxidative stress, inflammation, and altered cholesterol metabolism. The brain's high lipid content makes it particularly vulnerable to oxidative species, with the consequent enhancement of lipid peroxidation and cholesterol oxidation, and the subsequent formation of end products, mainly 4-hydroxynonenal and oxysterols, respectively from the two processes. The chronic inflammatory events observed in the AD brain include activation of microglia and astrocytes, together with enhancement of inflammatory molecule and free radical release. Along with glial cells, neurons themselves have been found to contribute to neuroinflammation in the AD brain, by serving as sources of inflammatory mediators. Oxidative stress is intimately associated with neuroinflammation, and a vicious circle has been found to connect oxidative stress and inflammation in AD. Alongside oxidative stress and inflammation, altered cholesterol metabolism and hypercholesterolemia also significantly contribute to neuronal damage and to progression of AD. Increasing evidence is now consolidating the hypothesis that oxidized cholesterol is the driving force behind the development of AD, and that oxysterols are the link connecting the disease to altered cholesterol metabolism in the brain and hypercholesterolemia; this is because of the ability of oxysterols, unlike cholesterol, to cross the blood brain barrier (BBB). The key role of oxysterols in AD pathogenesis has been strongly supported by research pointing to their involvement in modulating neuroinflammation, Aβ accumulation, and cell death. This review highlights the key role played by cholesterol and oxysterols in the brain in AD pathogenesis.
Keywords: Alzheimer’s disease; inflammation; oxidative stress; oxidized cholesterol; oxysterols.
Figures
References
-
- Abbas N., Bednar I., Mix E., Marie S., Paterson D., Ljungberg A., et al. . (2002). Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J. Neuroimmunol. 126, 50–57. 10.1016/s0165-5728(02)00050-4 - DOI - PubMed
-
- Abildayeva K., Jansen P. J., Hirsch-Reinshagen V., Bloks V. W., Bakker A. H., Ramaekers F. C., et al. . (2006). 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281, 12799–12808. 10.1074/jbc.m601019200 - DOI - PubMed
-
- Ahonen L., Maire F. B., Savolainen M., Kopra J., Vreeken R. J., Hankemeier T., et al. . (2014). Analysis of oxysterols and vitamin D metabolites in mouse brain and cell line samples by ultra-high-performance liquid chromatography-atmospheric pressure photoionization-mass spectrometry. J. Chromatogr. A. 1364, 214–222. 10.1016/j.chroma.2014.08.088 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

