High rate copper and energy recovery in microbial fuel cells
- PMID: 26150802
- PMCID: PMC4473641
- DOI: 10.3389/fmicb.2015.00527
High rate copper and energy recovery in microbial fuel cells
Abstract
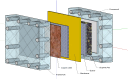
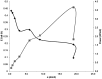
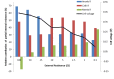
Bioelectrochemical systems (BESs) are a novel, promising technology for the recovery of metals. The prerequisite for upscaling from laboratory to industrial size is that high current and high power densities can be produced. In this study we report the recovery of copper from a copper sulfate stream (2 g L(-1) Cu(2+)) using a laboratory scale BES at high rate. To achieve this, we used a novel cell configuration to reduce the internal voltage losses of the system. At the anode, electroactive microorganisms produce electrons at the surface of an electrode, which generates a stable cell voltage of 485 mV when combined with a cathode where copper is reduced. In this system, a maximum current density of 23 A m(-2) in combination with a power density of 5.5 W m(-2) was produced. XRD analysis confirmed 99% purity in copper of copper deposited onto cathode surface. Analysis of voltage losses showed that at the highest current, most voltage losses occurred at the cathode, and membrane, while anode losses had the lowest contribution to the total voltage loss. These results encourage further development of BESs for bioelectrochemical metal recovery.
Keywords: bioelectrochemical systems; copper; metal recovery; microbial fuel cells.
Figures


References
-
- Alvarado S. (2002). Long term energy-related environmental issues of copper production. Energy 27 183–196. 10.1016/S0360-5442(01)00067-6 - DOI
-
- Foley J. M., Rozendal R. A., Hertle C. K., Lant P. A., Rabaey K. (2010). Life Cycle Assessment of High-Rate Anaerobic Treatment, Microbial Fuel Cells, and Microbial Electrolysis Cells. Available at: http://pubs.acs.org.ezproxy.library.wur.nl/doi/full/10.1021/es100125h [accessed March 26, 2015]. - DOI - PubMed
-
- Geman H., Smith W. O. (2013). Theory of storage, inventory and volatility in the LME base metals. Resour. Policy 38 18–28. 10.1016/j.resourpol.2012.06.014 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

