Multivalent polyglycerol supported imidazolidin-4-one organocatalysts for enantioselective Friedel-Crafts alkylations
- PMID: 26150897
- PMCID: PMC4463494
- DOI: 10.3762/bjoc.11.83
Multivalent polyglycerol supported imidazolidin-4-one organocatalysts for enantioselective Friedel-Crafts alkylations
Abstract
The first immobilization of a MacMillan's first generation organocatalyst onto dendritic support is described. A modified tyrosine-based imidazolidin-4-one was grafted to a soluble high-loading hyperbranched polyglycerol via a copper-catalyzed alkyne-azide cycloaddition (CuAAC) reaction and readily purified by dialysis. The efficiency of differently functionalized multivalent organocatalysts 4a-c was tested in the asymmetric Friedel-Crafts alkylation of N-methylpyrrole with α,β-unsaturated aldehydes. A variety of substituted enals was investigated to explore the activity of the catalytic system which was also compared with monovalent analogues. The catalyst 4b showed excellent turnover rates and no loss of activity due to immobilization, albeit moderate enantioselectivities were observed. Moreover, easy recovery by selective precipitation allowed the reuse of the catalyst for three cycles.
Keywords: Friedel–Crafts; homogeneous catalysis; hyperbranched polyglycerol; imidazolidin-4-one; multivalency.
Figures

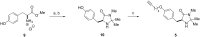
Similar articles
-
A click strategy for the immobilization of MacMillan organocatalysts onto polymers and magnetic nanoparticles.Org Lett. 2012 Jul 20;14(14):3668-71. doi: 10.1021/ol301515d. Epub 2012 Jul 3. Org Lett. 2012. PMID: 22758605
-
Polystyrene or Magnetic Nanoparticles as Support in Enantioselective Organocatalysis? A Case Study in Friedel-Crafts Chemistry.Org Lett. 2016 Apr 1;18(7):1602-5. doi: 10.1021/acs.orglett.6b00462. Epub 2016 Mar 24. Org Lett. 2016. PMID: 27010999
-
3-Mono-Substituted BINOL Phosphoric Acids as Effective Organocatalysts in Direct Enantioselective Friedel-Crafts-Type Alkylation of N-Unprotected α-Ketiminoester.Chemistry. 2018 Oct 12;24(57):15211-15214. doi: 10.1002/chem.201804078. Epub 2018 Sep 10. Chemistry. 2018. PMID: 30098059
-
Development of cascade reactions for the concise construction of diverse heterocyclic architectures.Acc Chem Res. 2012 Aug 21;45(8):1278-93. doi: 10.1021/ar200338s. Epub 2012 May 11. Acc Chem Res. 2012. PMID: 22577988 Review.
-
Asymmetric electrophilic functionalization of amino-substituted heteroaromatic compounds: a convenient tool for the enantioselective synthesis of nitrogen heterocycles.Chem Commun (Camb). 2024 Oct 22;60(85):12270-12286. doi: 10.1039/d4cc03680h. Chem Commun (Camb). 2024. PMID: 39351928 Review.
Cited by
-
Multivalency as a chemical organization and action principle.Beilstein J Org Chem. 2015 May 19;11:848-9. doi: 10.3762/bjoc.11.94. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26124885 Free PMC article. No abstract available.
-
Styrylpyridinium Derivatives for Fluorescent Cell Imaging.Pharmaceuticals (Basel). 2023 Sep 4;16(9):1245. doi: 10.3390/ph16091245. Pharmaceuticals (Basel). 2023. PMID: 37765053 Free PMC article.
-
Chiral GAP catalysts of phosphonylated imidazolidinones and their applications in asymmetric Diels-Alder and Friedel-Crafts reactions.Org Biomol Chem. 2017 Feb 21;15(7):1718-1724. doi: 10.1039/c6ob02801b. Epub 2017 Jan 31. Org Biomol Chem. 2017. PMID: 28139798 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
