A Randomized, Placebo-controlled Trial of Roflumilast. Effect on Proline-Glycine-Proline and Neutrophilic Inflammation in Chronic Obstructive Pulmonary Disease
- PMID: 26151090
- PMCID: PMC4642210
- DOI: 10.1164/rccm.201503-0543OC
A Randomized, Placebo-controlled Trial of Roflumilast. Effect on Proline-Glycine-Proline and Neutrophilic Inflammation in Chronic Obstructive Pulmonary Disease
Abstract
Rationale: Roflumilast is a therapeutic agent in the treatment of chronic obstructive pulmonary disease (COPD). It has antiinflammatory effects; however, it is not known whether it can affect a biologic pathway implicated in COPD pathogenesis and progression. The self-propagating acetyl-proline-glycine-proline (AcPGP) pathway is a novel means of neutrophilic inflammation that is pathologic in the development of COPD. AcPGP is produced by extracellular matrix collagen breakdown with prolyl endopeptidase and leukotriene A4 hydrolase serving as the enzymes responsible for its production and degradation, respectively.
Objectives: We hypothesized that roflumilast would decrease AcPGP, halting the feed-forward cycle of inflammation.
Methods: We conducted a single-center, placebo-controlled, randomized study investigating 12 weeks of roflumilast treatment added to current therapy in moderate-to-severe COPD with chronic bronchitis. Subjects underwent sputum and blood analyses, pulmonary function testing, exercise tolerance, and quality-of-life assessment at 0, 4, and 12 weeks.
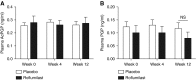
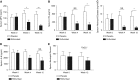
Measurements and main results: Twenty-seven patients were enrolled in the intention-to-treat analysis. Roflumilast treatment decreased sputum AcPGP by more than 50% (P < 0.01) and prolyl endopeptidase by 46% (P = 0.02), without significant improvement in leukotriene A4 hydrolase activity compared with placebo. Roflumilast also reduces other inflammatory markers. There were no significant changes in lung function, quality of life, or exercise tolerance between roflumilast- and placebo-treated groups.
Conclusions: Roflumilast reduces pulmonary inflammation through decreasing prolyl endopeptidase activity and AcPGP. As expected for lower AcPGP levels, markers of neutrophilic inflammation are blunted. Inhibiting this self-propagating pathway lessens the overall inflammatory burden, which may alter the natural history of COPD, including the risk of exacerbation. Clinical trial registered with www.clinicaltrials.gov (NCT 01572948).
Trial registration: ClinicalTrials.gov NCT01572948.
Keywords: COPD; neutrophil; proline-glycine-proline; prolyl endopeptidase; roflumilast.
Figures


References
-
- Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2013;61:1–117. - PubMed
-
- Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, Calverley P, Coxson H, Crim C, Edwards LD, et al. ECLIPSE Investigators. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065–1072. - PubMed
-
- Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002;121(Suppl. 5):151S–155S. - PubMed
-
- Mannino DM. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care. 2003;48:1185–1191; discussion 1191–1183. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

