Multiscale biomechanical responses of adapted bone-periodontal ligament-tooth fibrous joints
- PMID: 26151121
- PMCID: PMC4663099
- DOI: 10.1016/j.bone.2015.07.004
Multiscale biomechanical responses of adapted bone-periodontal ligament-tooth fibrous joints
Abstract
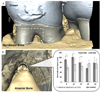
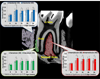
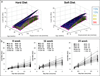
Reduced functional loads cause adaptations in organs. In this study, temporal adaptations of bone-ligament-tooth fibrous joints to reduced functional loads were mapped using a holistic approach. Systematic studies were performed to evaluate organ-level and tissue-level adaptations in specimens harvested periodically from rats (N=60) given powder food for 6 months over 8,12,16,20, and 24 weeks. Bone-periodontal ligament (PDL)-tooth fibrous joint adaptation was evaluated by comparing changes in joint stiffness with changes in functional space between the tooth and alveolar bony socket. Adaptations in tissues included mapping changes in the PDL and bone architecture as observed from collagen birefringence, bone hardness and volume fraction in rats fed soft foods (soft diet, SD) compared to those fed hard pellets as a routine diet (hard diet, HD). In situ biomechanical testing on harvested fibrous joints revealed increased stiffness in SD groups (SD:239-605 N/mm) (p<0.05) at 8 and 12 weeks. Increased joint stiffness in early development phase was due to decreased functional space (at 8 weeks change in functional space was -33 μm, at 12 weeks change in functional space was -30 μm) and shifts in tissue quality as highlighted by birefringence, architecture and hardness. These physical changes were not observed in joints that were well into function, that is, in rodents older than 12 weeks of age. Significant adaptations in older groups were highlighted by shifts in bone growth (bone volume fraction 24 weeks: Δ-0.06) and bone hardness (8 weeks: Δ-0.04 GPa, 16 weeks: Δ-0.07 GPa, 24 weeks: Δ-0.06 GPa). The response rate (N/s) of joints to mechanical loads decreased in SD groups. Results from the study showed that joint adaptation depended on age. The initial form-related adaptation (observed change in functional space) can challenge strain-adaptive nature of tissues to meet functional demands with increasing age into adulthood. The coupled effect between functional space in the bone-PDL-tooth complex and strain-adaptive nature of tissues is necessary to accommodate functional demands, and is temporally sensitive despite joint malfunction. From an applied science perspective, we propose that adaptations are registered as functional history in tissues and joints.
Keywords: Biomechanics; Bone–PDL–tooth fibrous joint; Multiscale; Periodontal ligament; Rat; Stiffness.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Reduced functional loads alter the physical characteristics of the bone-periodontal ligament-cementum complex.J Periodontal Res. 2011 Dec;46(6):730-41. doi: 10.1111/j.1600-0765.2011.01396.x. Epub 2011 Aug 17. J Periodontal Res. 2011. PMID: 21848615 Free PMC article.
-
Biomechanical pathways of dentoalveolar fibrous joints in health and disease.Periodontol 2000. 2020 Feb;82(1):238-256. doi: 10.1111/prd.12306. Periodontol 2000. 2020. PMID: 31850635 Review.
-
Functional Adaptation of LPS-affected Dentoalveolar Fibrous Joints in Rats.J Periodontal Res. 2022 Jan;57(1):131-141. doi: 10.1111/jre.12946. Epub 2021 Nov 28. J Periodontal Res. 2022. PMID: 34839547
-
Biomechanical adaptation of the bone-periodontal ligament (PDL)-tooth fibrous joint as a consequence of disease.J Biomech. 2014 Jun 27;47(9):2102-14. doi: 10.1016/j.jbiomech.2013.10.059. Epub 2013 Nov 8. J Biomech. 2014. PMID: 24332618 Free PMC article.
-
A Force on the Crown and Tug of War in the Periodontal Complex.J Dent Res. 2018 Mar;97(3):241-250. doi: 10.1177/0022034517744556. Epub 2018 Jan 24. J Dent Res. 2018. PMID: 29364757 Free PMC article. Review.
Cited by
-
Spatial Platform for Periodontal Ligament Angulation and Regeneration: In Vivo Pilot Study.J Funct Biomater. 2025 Mar 13;16(3):99. doi: 10.3390/jfb16030099. J Funct Biomater. 2025. PMID: 40137378 Free PMC article.
-
Association of feeding behavior with jaw bone metabolism and tongue pressure.Jpn Dent Sci Rev. 2018 Nov;54(4):174-182. doi: 10.1016/j.jdsr.2018.05.001. Epub 2018 Sep 3. Jpn Dent Sci Rev. 2018. PMID: 30302136 Free PMC article. Review.
-
Nonuniformity in Periodontal Ligament: Mechanics and Matrix Composition.J Dent Res. 2021 Feb;100(2):179-186. doi: 10.1177/0022034520962455. Epub 2020 Oct 10. J Dent Res. 2021. PMID: 33043806 Free PMC article.
-
Tensile testing of the mechanical behavior of the human periodontal ligament.Biomed Eng Online. 2018 Nov 23;17(1):172. doi: 10.1186/s12938-018-0607-0. Biomed Eng Online. 2018. PMID: 30470224 Free PMC article.
-
Functional adaptation of interradicular alveolar bone to reduced chewing loads on dentoalveolar joints in rats.Dent Mater. 2021 Mar;37(3):486-495. doi: 10.1016/j.dental.2020.12.003. Epub 2021 Feb 12. Dent Mater. 2021. PMID: 33589268 Free PMC article.
References
-
- Carter DR, Van der Meulen MCH, Beaupré GS. Mechanical factors in bone growth and development. Bone. 1996;18:S5–S10. - PubMed
-
- Carter DR. Mechanical loading histories and cortical bone remodeling. Calcif. Tissue Int. 1984;36:S19–S24. - PubMed
-
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. - PubMed
-
- Posner AS. Crystal chemistry of bone mineral. Physiol Rev. 1969;49:760–792. - PubMed
-
- Dawson PE. Functional occlusion: from TMJ to smile design. Elsevier Health Sciences. 2006
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

