SUMO and ubiquitin-dependent XPC exchange drives nucleotide excision repair
- PMID: 26151477
- PMCID: PMC4501428
- DOI: 10.1038/ncomms8499
SUMO and ubiquitin-dependent XPC exchange drives nucleotide excision repair
Abstract
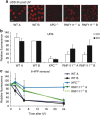
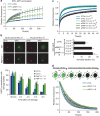
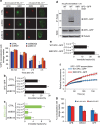
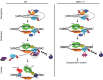
XPC recognizes UV-induced DNA lesions and initiates their removal by nucleotide excision repair (NER). Damage recognition in NER is tightly controlled by ubiquitin and SUMO modifications. Recent studies have shown that the SUMO-targeted ubiquitin ligase RNF111 promotes K63-linked ubiquitylation of SUMOylated XPC after DNA damage. However, the exact regulatory function of these modifications in vivo remains elusive. Here we show that RNF111 is required for efficient repair of ultraviolet-induced DNA lesions. RNF111-mediated ubiquitylation promotes the release of XPC from damaged DNA after NER initiation, and is needed for stable incorporation of the NER endonucleases XPG and ERCC1/XPF. Our data suggest that RNF111, together with the CRL4(DDB2) ubiquitin ligase complex, is responsible for sequential XPC ubiquitylation, which regulates the recruitment and release of XPC and is crucial for efficient progression of the NER reaction, thereby providing an extra layer of quality control of NER.
Figures

References
-
- Marteijn J. A., Lans H., Vermeulen W. & Hoeijmakers J. H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 15, 465–481 (2014). - PubMed
-
- Yokoi M. et al.. The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J. Biol. Chem. 275, 9870–9875 (2000). - PubMed
-
- Volker M. et al.. Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell 8, 213–224 (2001). - PubMed
-
- Camenisch U., Dip R., Schumacher S. B., Schuler B. & Naegeli H. Recognition of helical kinks by xeroderma pigmentosum group A protein triggers DNA excision repair. Nat. Struct. Mol. Biol. 13, 278–284 (2006). - PubMed
-
- Sugasawa K., Akagi J., Nishi R., Iwai S. & Hanaoka F. Two-step recognition of DNA damage for mammalian nucleotide excision repair: directional binding of the XPC complex and DNA strand scanning. Mol. Cell 36, 642–653 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

