Kindlin-2 controls TGF-β signalling and Sox9 expression to regulate chondrogenesis
- PMID: 26151572
- PMCID: PMC4498276
- DOI: 10.1038/ncomms8531
Kindlin-2 controls TGF-β signalling and Sox9 expression to regulate chondrogenesis
Abstract
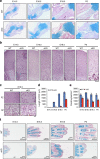
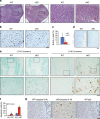
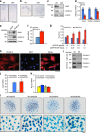
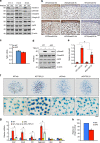
The signals that control skeletogenesis are incompletely understood. Here we show that deleting Kindlin-2 in Prx1-expressing mesenchymal progenitors in mice causes neonatal lethality, chondrodysplasia and loss of the skull vault. Kindlin-2 ablation reduces chondrocyte density by decreasing cell proliferation and increasing apoptosis, and disrupts column formation, thus impairing the formation of the primary ossification center and causing severe limb shortening. Remarkably, Kindlin-2 localizes to not only focal adhesions, but also to the nuclei of chondrocytes. Loss of Kindlin-2 reduces, while the overexpression of Kindlin-2 increases, Sox9 expression. Furthermore, the overexpression of Sox9 restores the defects in chondrogenic differentiation induced by Kindlin-2 deletion in vitro. In addition, Kindlin-2 ablation inhibits TGF-β1-induced Smad2 phosphorylation and chondrocyte differentiation. Finally, deleting Kindlin-2 in chondrocytes directly impairs chondrocyte functions, resulting in progressive dwarfism and kyphosis in mice. These studies uncover a previously unrecognized function for Kindlin-2 and a mechanism for regulation of the chondrocyte differentiation programme and chondrogenesis.
Figures

Similar articles
-
New target genes for NOV/CCN3 in chondrocytes: TGF-beta2 and type X collagen.J Bone Miner Res. 2005 Dec;20(12):2213-23. doi: 10.1359/JBMR.050818. Epub 2005 Aug 22. J Bone Miner Res. 2005. PMID: 16294274
-
Transforming Growth Factor-β-Induced KDM4B Promotes Chondrogenic Differentiation of Human Mesenchymal Stem Cells.Stem Cells. 2016 Mar;34(3):711-9. doi: 10.1002/stem.2231. Epub 2015 Nov 17. Stem Cells. 2016. PMID: 26485430 Free PMC article.
-
Runx2 regulates endochondral ossification through control of chondrocyte proliferation and differentiation.J Bone Miner Res. 2014 Dec;29(12):2653-65. doi: 10.1002/jbmr.2287. J Bone Miner Res. 2014. PMID: 24862038 Free PMC article.
-
Histone acetylation influences the activity of Sox9-related transcriptional complex.Acta Med Okayama. 2010 Dec;64(6):351-7. doi: 10.18926/AMO/41320. Acta Med Okayama. 2010. PMID: 21173804 Review.
-
SOX9 and the many facets of its regulation in the chondrocyte lineage.Connect Tissue Res. 2017 Jan;58(1):2-14. doi: 10.1080/03008207.2016.1183667. Epub 2016 Apr 29. Connect Tissue Res. 2017. PMID: 27128146 Free PMC article. Review.
Cited by
-
Integrin β1 Promotes Pancreatic Tumor Growth by Upregulating Kindlin-2 and TGF-β Receptor-2.Int J Mol Sci. 2021 Sep 30;22(19):10599. doi: 10.3390/ijms221910599. Int J Mol Sci. 2021. PMID: 34638957 Free PMC article.
-
Phosphatidylinositol 3-Kinase/Akt Mediates Integrin Signaling To Control RNA Polymerase I Transcriptional Activity.Mol Cell Biol. 2016 May 2;36(10):1555-68. doi: 10.1128/MCB.00004-16. Print 2016 May 15. Mol Cell Biol. 2016. PMID: 26976639 Free PMC article.
-
The Evolving Complexity of the Podocyte Cytoskeleton.J Am Soc Nephrol. 2017 Nov;28(11):3166-3174. doi: 10.1681/ASN.2017020143. Epub 2017 Sep 1. J Am Soc Nephrol. 2017. PMID: 28864466 Free PMC article. Review.
-
Integrin signalling in joint development, homeostasis and osteoarthritis.Nat Rev Rheumatol. 2024 Aug;20(8):492-509. doi: 10.1038/s41584-024-01130-8. Epub 2024 Jul 16. Nat Rev Rheumatol. 2024. PMID: 39014254 Free PMC article. Review.
-
Progress of orthopaedic research in China over the last decade.J Orthop Translat. 2020 May 16;24:131-137. doi: 10.1016/j.jot.2020.04.010. eCollection 2020 Sep. J Orthop Translat. 2020. PMID: 32913711 Free PMC article.
References
-
- Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 13, 27–38 (2012). - PubMed
-
- Mackie E. J., Ahmed Y. A., Tatarczuch L., Chen K. S. & Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 40, 46–62 (2008). - PubMed
-
- de Crombrugghe B. et al.. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 19, 389–394 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AR067935/AR/NIAMS NIH HHS/United States
- R01 AR068950/AR/NIAMS NIH HHS/United States
- R01 AR062136/AR/NIAMS NIH HHS/United States
- AR064874/AR/NIAMS NIH HHS/United States
- R01 DK072230/DK/NIDDK NIH HHS/United States
- R01 AR054465/AR/NIAMS NIH HHS/United States
- AR068950/AR/NIAMS NIH HHS/United States
- R01 GM065188/GM/NIGMS NIH HHS/United States
- R01 AR057310/AR/NIAMS NIH HHS/United States
- AR053220/AR/NIAMS NIH HHS/United States
- R21 AR064874/AR/NIAMS NIH HHS/United States
- R01 AR059647/AR/NIAMS NIH HHS/United States
- GM65188/GM/NIGMS NIH HHS/United States
- AR059647/AR/NIAMS NIH HHS/United States
- R01 AR053220/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

