Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways
- PMID: 26151844
- PMCID: PMC4519888
- DOI: 10.3390/ijms160715086
Retinal Cell Death Caused by Sodium Iodate Involves Multiple Caspase-Dependent and Caspase-Independent Cell-Death Pathways
Abstract
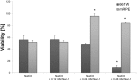
Herein, we have investigated retinal cell-death pathways in response to the retina toxin sodium iodate (NaIO3) both in vivo and in vitro. C57/BL6 mice were treated with a single intravenous injection of NaIO3 (35 mg/kg). Morphological changes in the retina post NaIO3 injection in comparison to untreated controls were assessed using electron microscopy. Cell death was determined by TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining. The activation of caspases and calpain was measured using immunohistochemistry. Additionally, cytotoxicity and apoptosis in retinal pigment epithelial (RPE) cells, primary retinal cells, and the cone photoreceptor (PRC) cell line 661W were assessed in vitro after NaIO3 treatment using the ApoToxGlo™ assay. The 7-AAD/Annexin-V staining was performed and necrostatin (Nec-1) was administered to the NaIO3-treated cells to confirm the results. In vivo, degenerating RPE cells displayed a rounded shape and retracted microvilli, whereas PRCs featured apoptotic nuclei. Caspase and calpain activity was significantly upregulated in retinal sections and protein samples from NaIO3-treated animals. In vitro, NaIO3 induced necrosis in RPE cells and apoptosis in PRCs. Furthermore, Nec-1 significantly decreased NaIO3-induced RPE cell death, but had no rescue effect on treated PRCs. In summary, several different cell-death pathways are activated in retinal cells as a result of NaIO3.
Keywords: apoptosis; cell death; in vitro; in vivo; necrosis; photoreceptors; retinal pigment epithelium; sodium iodate.
Figures



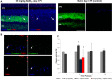

 cells,
cells,  + 1 μM staurosporine,
+ 1 μM staurosporine,  + 100% sonication,
+ 100% sonication,  + 6 mM NaIO3,
+ 6 mM NaIO3,  + 12 mM NaIO3,
+ 12 mM NaIO3,  + 48 mM NaIO3); *
p < 0.05.
+ 48 mM NaIO3); *
p < 0.05.
Similar articles
-
Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3476-3487. doi: 10.1167/iovs.17-23532. Invest Ophthalmol Vis Sci. 2018. PMID: 30025075
-
CAMK2D and Complement Factor I-Involved Calcium/Calmodulin Signaling Modulates Sodium Iodate-Induced Mouse Retinal Degeneration.Invest Ophthalmol Vis Sci. 2025 Jan 2;66(1):63. doi: 10.1167/iovs.66.1.63. Invest Ophthalmol Vis Sci. 2025. PMID: 39873650 Free PMC article.
-
What Can Pharmacological Models of Retinal Degeneration Tell Us?Curr Mol Med. 2017;17(2):100-107. doi: 10.2174/1566524017666170331162048. Curr Mol Med. 2017. PMID: 28429669 Review.
-
Dose-dependent retinal changes following sodium iodate administration: application of spectral-domain optical coherence tomography for monitoring of retinal injury and endogenous regeneration.Curr Eye Res. 2014 Oct;39(10):1033-41. doi: 10.3109/02713683.2014.892996. Epub 2014 Mar 24. Curr Eye Res. 2014. PMID: 24661221
-
Regulated cell death pathways in the sodium iodate model: Insights and implications for AMD.Exp Eye Res. 2024 Jan;238:109728. doi: 10.1016/j.exer.2023.109728. Epub 2023 Nov 14. Exp Eye Res. 2024. PMID: 37972750 Free PMC article. Review.
Cited by
-
Multi-Wavelength Photobiomodulation Ameliorates Sodium Iodate-Induced Age-Related Macular Degeneration in Rats.Int J Mol Sci. 2023 Dec 12;24(24):17394. doi: 10.3390/ijms242417394. Int J Mol Sci. 2023. PMID: 38139223 Free PMC article.
-
Iodate induced toxic retinopathy: a case report.Int J Retina Vitreous. 2018 Aug 1;4:27. doi: 10.1186/s40942-018-0130-2. eCollection 2018. Int J Retina Vitreous. 2018. PMID: 30083392 Free PMC article.
-
Oxidative Model of Retinal Neurodegeneration Induced by Sodium Iodate: Morphofunctional Assessment of the Visual Pathway.Antioxidants (Basel). 2023 Aug 10;12(8):1594. doi: 10.3390/antiox12081594. Antioxidants (Basel). 2023. PMID: 37627589 Free PMC article.
-
Inhibition of thyroid hormone signaling protects retinal pigment epithelium and photoreceptors from cell death in a mouse model of age-related macular degeneration.Cell Death Dis. 2020 Jan 13;11(1):24. doi: 10.1038/s41419-019-2216-7. Cell Death Dis. 2020. PMID: 31932580 Free PMC article.
-
Application of CRISPR Tools for Variant Interpretation and Disease Modeling in Inherited Retinal Dystrophies.Genes (Basel). 2020 Apr 27;11(5):473. doi: 10.3390/genes11050473. Genes (Basel). 2020. PMID: 32349249 Free PMC article. Review.
References
-
- Webster S.H., Rice M.E., Highman B., von Oettingen W.F. The toxicology of potassium and sodium iodates: Acute toxicity in mice. J. Pharmacol. Exp. Ther. 1957;120:171–178. - PubMed
-
- Higuchi M., Tomioka M., Takano J., Shirotani K., Iwata N., Masumoto H., Maki M., Itohara S., Saido T.C. Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice overexpressing their specific inhibitors. J. Biol. Chem. 2005;280:15229–15237. doi: 10.1074/jbc.M500939200. - DOI - PubMed
-
- Machalinska A., Kawa M.P., Pius-Sadowska E., Roginska D., Klos P., Baumert B., Wiszniewska B., Machalinski B. Endogenous regeneration of damaged retinal pigment epithelium following low dose sodium iodate administration: An insight into the role of glial cells in retinal repair. Exp. Eye Res. 2013;112:68–78. doi: 10.1016/j.exer.2013.04.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

