Viral expression of ALS-linked ubiquilin-2 mutants causes inclusion pathology and behavioral deficits in mice
- PMID: 26152284
- PMCID: PMC4495639
- DOI: 10.1186/s13024-015-0026-7
Viral expression of ALS-linked ubiquilin-2 mutants causes inclusion pathology and behavioral deficits in mice
Abstract
Background: UBQLN2 mutations have recently been associated with familial forms of amyotrophic lateral sclerosis (ALS) and ALS-dementia. UBQLN2 encodes for ubiquilin-2, a member of the ubiquitin-like protein family which facilitates delivery of ubiquitinated proteins to the proteasome for degradation. To study the potential role of ubiquilin-2 in ALS, we used recombinant adeno-associated viral (rAAV) vectors to express UBQLN2 and three of the identified ALS-linked mutants (P497H, P497S, and P506T) in primary neuroglial cultures and in developing neonatal mouse brains.
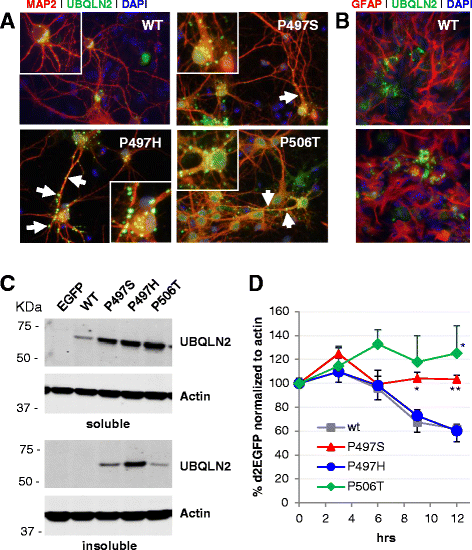
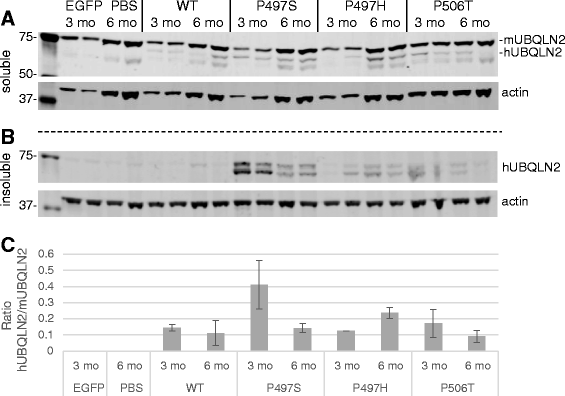
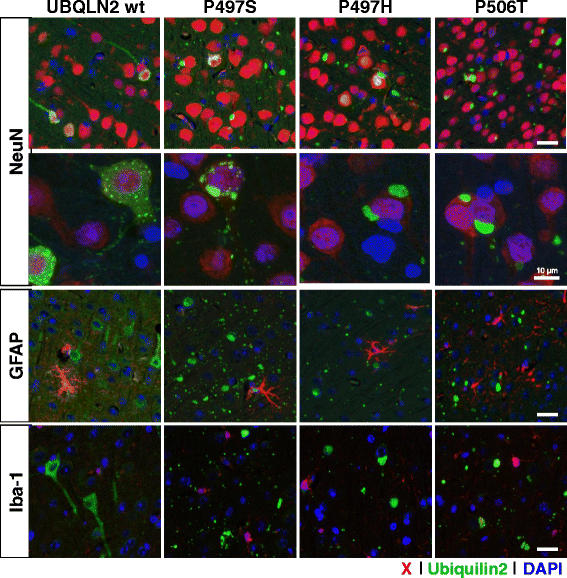
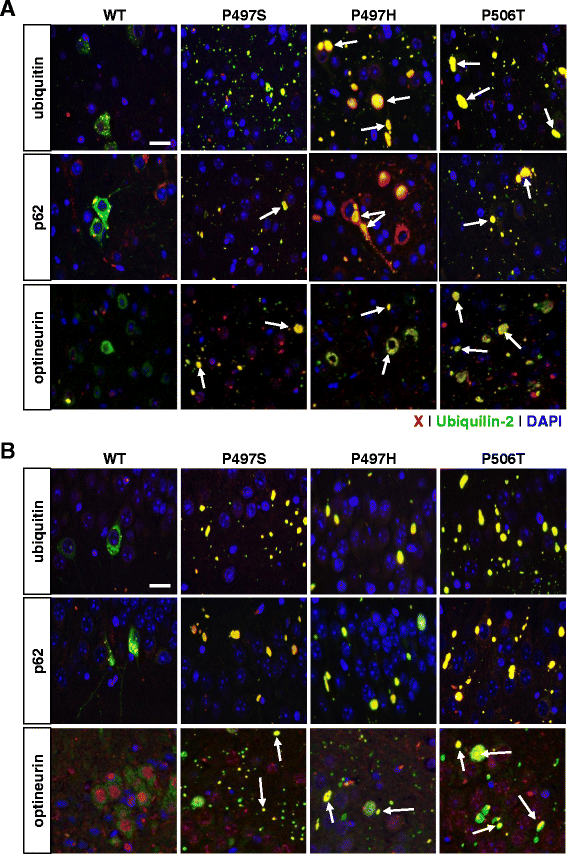
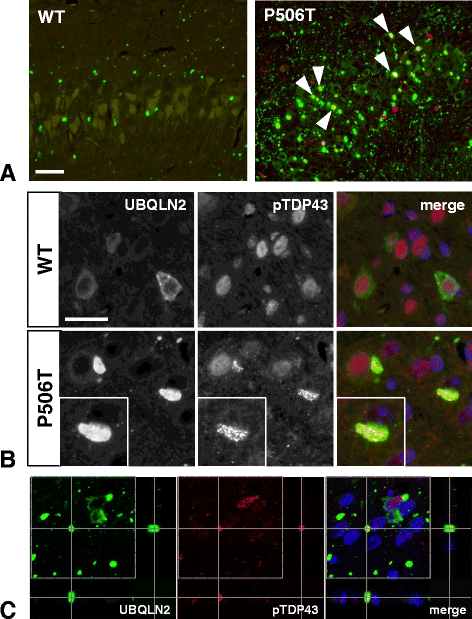
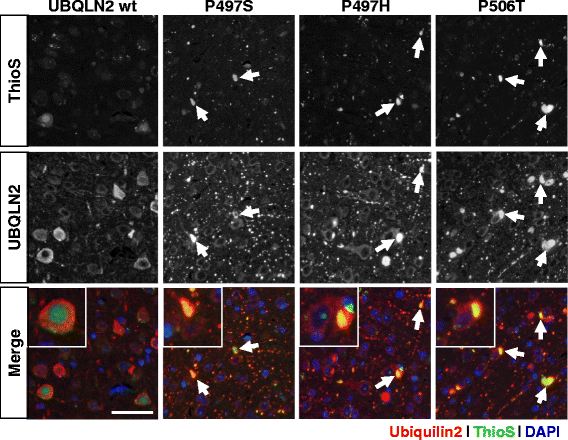
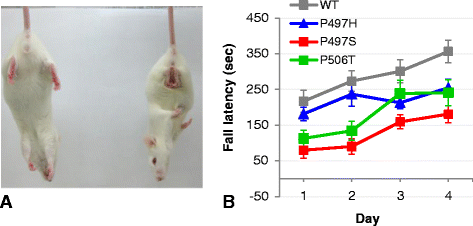
Results: In primary cultures rAAV2/8-mediated expression of UBQLN2 mutants resulted in inclusion bodies and insoluble aggregates. Intracerebroventricular injection of FVB mice at post-natal day 0 with rAAV2/8 expressing wild type or mutant UBQLN2 resulted in widespread, sustained expression of ubiquilin-2 in brain. In contrast to wild type, mutant UBQLN2 expression induced significant pathology with large neuronal, cytoplasmic inclusions and ubiquilin-2-positive aggregates in surrounding neuropil. Ubiquilin-2 inclusions co-localized with ubiquitin, p62/SQSTM, optineurin, and occasionally TDP-43, but were negative for α-synuclein, neurofilament, tau, and FUS. Mutant UBLQN2 expression also resulted in Thioflavin-S-positive inclusions/aggregates. Mice expressing mutant forms of UBQLN2 variably developed a motor phenotype at 3-4 months, including nonspecific clasping and rotarod deficits.
Conclusions: These findings demonstrate that UBQLN2 mutants (P497H, P497S, and P506T) induce proteinopathy and cause behavioral deficits, supporting a "toxic" gain-of-function, which may contribute to ALS pathology. These data establish also that our rAAV model can be used to rapidly assess the pathological consequences of various UBQLN2 mutations and provides an agile system to further interrogate the molecular mechanisms of ubiquilins in neurodegeneration.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

