Platelet-Synthesized Testosterone in Men with Prostate Cancer Induces Androgen Receptor Signaling
- PMID: 26152357
- PMCID: PMC4719002
- DOI: 10.1016/j.neo.2015.05.003
Platelet-Synthesized Testosterone in Men with Prostate Cancer Induces Androgen Receptor Signaling
Abstract
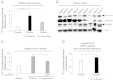
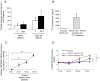
Platelets have been long postulated to play a critical role in the pathogenesis of prostate cancer, although relatively little is known regarding the precise mechanisms involved. Androgen deprivation therapy (ADT) for prostate cancer eventually fails with relapse occurring in the form of castration-resistant prostate cancer (CRPC). CRPC tumors typically overexpress androgen receptor (AR), demonstrating continued dependence upon AR signaling. Platelets have been previously demonstrated to contain androgens, and we sought to explore the contribution of platelet-derived androgens in CRPC. In this study, we examined the role of platelet-derived androgens in vitro using platelets from men with CRPC, men with high-risk prostate cancer, and healthy male donors. A series of in vitro assays was performed to elucidate the impact of platelet-derived androgens on androgen-sensitive prostate tumor cells. By examining platelet-derived androgen effects on AR signaling in prostate tumor cells, we found that platelets, from men with CRPC and on ADT, strongly induce AR target genes and tumor cell proliferation. Moreover, we show a fully intact testosterone (T) biosynthetic pathway within platelets from its precursor cholesterol and demonstrate that platelets of CRPC patients with ADT resistance are able to generate T. Overall, our findings reveal an unknown capacity of platelets to synthesize T at functionally relevant levels in patients with lethal prostate cancer. Importantly, it suggests a novel paracrine mechanism of T production that may act to sustain CRPC state and potentiate therapeutic resistance.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Sulforaphane increases the efficacy of anti-androgens by rapidly decreasing androgen receptor levels in prostate cancer cells.Int J Oncol. 2016 Oct;49(4):1609-19. doi: 10.3892/ijo.2016.3641. Epub 2016 Aug 2. Int J Oncol. 2016. PMID: 27499349
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Resistance to docetaxel in prostate cancer is associated with androgen receptor activation and loss of KDM5D expression.Proc Natl Acad Sci U S A. 2016 May 31;113(22):6259-64. doi: 10.1073/pnas.1600420113. Epub 2016 May 16. Proc Natl Acad Sci U S A. 2016. PMID: 27185910 Free PMC article.
-
Androgen receptor co-regulatory networks in castration-resistant prostate cancer.Endocr Relat Cancer. 2013 Dec 16;21(1):R1-R11. doi: 10.1530/ERC-13-0326. Print 2014 Feb. Endocr Relat Cancer. 2013. PMID: 24152433 Review.
-
Identification of an anabolic selective androgen receptor modulator that actively induces death of androgen-independent prostate cancer cells.J Steroid Biochem Mol Biol. 2014 Sep;143:29-39. doi: 10.1016/j.jsbmb.2014.02.005. Epub 2014 Feb 22. J Steroid Biochem Mol Biol. 2014. PMID: 24565564
Cited by
-
Thrombo-inflammation linking androgen suppression with cardiovascular risk in patients with prostate cancer.Cardiooncology. 2024 Dec 5;10(1):87. doi: 10.1186/s40959-024-00278-2. Cardiooncology. 2024. PMID: 39639392 Free PMC article. Review.
-
Testosterone accumulation in prostate cancer cells is enhanced by facilitated diffusion.Prostate. 2019 Sep;79(13):1530-1542. doi: 10.1002/pros.23874. Epub 2019 Aug 2. Prostate. 2019. PMID: 31376206 Free PMC article.
-
Platelet PD-L1 suppresses anti-cancer immune cell activity in PD-L1 negative tumors.Sci Rep. 2020 Nov 9;10(1):19296. doi: 10.1038/s41598-020-76351-4. Sci Rep. 2020. PMID: 33168847 Free PMC article.
-
The dynamic role of platelets in cancer progression and their therapeutic implications.Nat Rev Cancer. 2024 Jan;24(1):72-87. doi: 10.1038/s41568-023-00639-6. Epub 2023 Dec 1. Nat Rev Cancer. 2024. PMID: 38040850 Review.
-
Molecular landscape for risk prediction and personalized therapeutics of castration-resistant prostate cancer: at a glance.Front Endocrinol (Lausanne). 2024 Jun 3;15:1360430. doi: 10.3389/fendo.2024.1360430. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38887275 Free PMC article. Review.
References
-
- Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol. 2012;30:644–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

