Chemorepulsion from the Quorum Signal Autoinducer-2 Promotes Helicobacter pylori Biofilm Dispersal
- PMID: 26152582
- PMCID: PMC4488943
- DOI: 10.1128/mBio.00379-15
Chemorepulsion from the Quorum Signal Autoinducer-2 Promotes Helicobacter pylori Biofilm Dispersal
Abstract
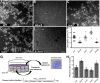
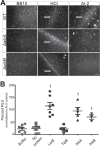
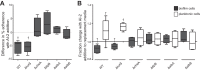
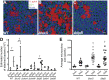
The gastric pathogen Helicobacter pylori forms biofilms on abiotic and biotic surfaces. We have shown previously that H. pylori perceives the quorum signal autoinducer-2 (AI-2) as a chemorepellent. We report here that H. pylori chemorepulsion from endogenous AI-2 influences the proportions and spatial organization of cells within biofilms. Strains that fail to produce AI-2 (∆luxS strains) or are defective for chemotaxis (∆cheA strains) formed more spatially homogenous biofilms with a greater proportion of adherent versus planktonic cells than wild-type biofilms. Reciprocally, a strain that overproduced AI-2 (luxS(OP)) formed biofilms with proportionally fewer adherent cells. Along with the known AI-2 chemoreceptor, TlpB, we identified AibA and AibB, two novel periplasmic binding proteins that are required for the AI-2 chemorepulsion response. Disruptions in any of the proteins required for AI-2 chemotaxis recapitulated the biofilm adherence and spatial organization phenotype of the ∆luxS mutant. Furthermore, exogenous administration of AI-2 was sufficient to decrease the proportion of adherent cells in biofilms and promote dispersal of cells from biofilms in a chemotaxis-dependent manner. Finally, we found that disruption of AI-2 production or AI-2 chemotaxis resulted in increased clustering of cells in microcolonies on cultured epithelial cells. We conclude that chemotaxis from AI-2 is a determinant of H. pylori biofilm spatial organization and dispersal.
Importance: Bacterial biofilms are ubiquitous in nature, but the mechanisms governing their assembly and spatial organization are not fully understood. Bacterial communication through quorum sensing has been shown to influence biofilm growth through the regulation of biofilm genes. Our study revealed a new role for quorum sensing in biofilms through rapid chemotactic responses to quorum signals. Specifically, we studied how chemorepulsion of Helicobacter pylori from the universal quorum signal autoinducer-2 (AI-2) shapes the spatial organization of its biofilms. We demonstrate that the chemorepulsive response of H. pylori to AI-2 is necessary to promote its dispersal from biofilms grown on both abiotic and biotic surfaces and is sufficient to promote dispersal in a chemotaxis-dependent manner. This work has broad implications for understanding the mechanisms by which endogenously produced microbial compounds shape the assembly and spatial organization of microbial communities in their environments.
Copyright © 2015 Anderson et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
