Cell-Type-Specific Cytokinin Distribution within the Arabidopsis Primary Root Apex
- PMID: 26152699
- PMCID: PMC4531351
- DOI: 10.1105/tpc.15.00176
Cell-Type-Specific Cytokinin Distribution within the Arabidopsis Primary Root Apex
Abstract
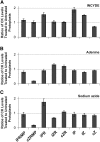
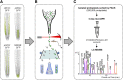
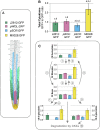
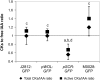
Cytokinins (CKs) play a crucial role in many physiological and developmental processes at the levels of individual plant components (cells, tissues, and organs) and by coordinating activities across these parts. High-resolution measurements of intracellular CKs in different plant tissues can therefore provide insights into their metabolism and mode of action. Here, we applied fluorescence-activated cell sorting of green fluorescent protein (GFP)-marked cell types, combined with solid-phase microextraction and an ultra-high-sensitivity mass spectrometry (MS) method for analysis of CK biosynthesis and homeostasis at cellular resolution. This method was validated by series of control experiments, establishing that protoplast isolation and cell sorting procedures did not greatly alter endogenous CK levels. The MS-based method facilitated the quantification of all the well known CK isoprenoid metabolites in four different transgenic Arabidopsis thaliana lines expressing GFP in specific cell populations within the primary root apex. Our results revealed the presence of a CK gradient within the Arabidopsis root tip, with a concentration maximum in the lateral root cap, columella, columella initials, and quiescent center cells. This distribution, when compared with previously published auxin gradients, implies that the well known antagonistic interactions between the two hormone groups are cell type specific.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures


Comment in
-
Measuring Cytokinin Levels in the Root Tip by the Zeptomole.Plant Cell. 2015 Jul;27(7):1819. doi: 10.1105/tpc.15.00553. Epub 2015 Jul 7. Plant Cell. 2015. PMID: 26152698 Free PMC article. No abstract available.
References
-
- Aremu A.O., Bairu M.W., Novák O., Plačková L., Zatloukal M., Doležal K., Finnie J.F., Strnad M., Van Staden J. (2012). Physiological responses and endogenous cytokinin profiles of tissue-cultured ‘Williams’ bananas in relation to roscovitine and an inhibitor of cytokinin oxidase/dehydrogenase (INCYDE) treatments. Planta 236: 1775–1790. - PubMed
-
- Birnbaum K., Jung J.W., Wang J.Y., Lambert G.M., Hirst J.A., Galbraith D.W., Benfey P.N. (2005). Cell type-specific expression profiling in plants via cell sorting of protoplasts from fluorescent reporter lines. Nat. Methods 2: 615–619. - PubMed
-
- Birnbaum K., Shasha D.E., Wang J.Y., Jung J.W., Lambert G.M., Galbraith D.W., Benfey P.N. (2003). A gene expression map of the Arabidopsis root. Science 302: 1956–1960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

