Catalytic Mechanisms of Fe(II)- and 2-Oxoglutarate-dependent Oxygenases
- PMID: 26152721
- PMCID: PMC4543632
- DOI: 10.1074/jbc.R115.648691
Catalytic Mechanisms of Fe(II)- and 2-Oxoglutarate-dependent Oxygenases
Abstract
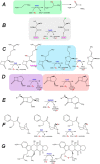
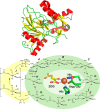
Mononuclear non-heme Fe(II)- and 2-oxoglutarate (2OG)-dependent oxygenases comprise a large family of enzymes that utilize an Fe(IV)-oxo intermediate to initiate diverse oxidative transformations with important biological roles. Here, four of the major types of Fe(II)/2OG-dependent reactions are detailed: hydroxylation, halogenation, ring formation, and desaturation. In addition, an atypical epimerization reaction is described. Studies identifying several key intermediates in catalysis are concisely summarized, and the proposed mechanisms are explained. In addition, a variety of other transformations catalyzed by selected family members are briefly described to further highlight the chemical versatility of these enzymes.
Keywords: 2-oxoglutarate; desaturation; dioxygenase; enzyme mechanism; halogenase; hydroxylase; iron; metalloenzyme; ring-formation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Abu-Omar M. M., Loaiza A., Hontzeas N. (2005) Reaction mechanisms of mononuclear non-heme iron oxygenases. Chem. Rev. 105, 2227–2252 - PubMed
-
- Decker A., Solomon E. I. (2005) Dioxygen activation by copper, heme and non-heme iron enzymes: comparison of electronic structures and reactivities. Curr. Opin. Chem. Biol. 9, 152–163 - PubMed
-
- Costas M., Mehn M. P., Jensen M. P., Que L., Jr. (2004) Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem. Rev. 104, 939–986 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

