Bortezomib, thalidomide and dexamethasone, with or without cyclophosphamide, for patients with previously untreated multiple myeloma: 5-year follow-up
- PMID: 26153365
- PMCID: PMC4758383
- DOI: 10.1111/bjh.13582
Bortezomib, thalidomide and dexamethasone, with or without cyclophosphamide, for patients with previously untreated multiple myeloma: 5-year follow-up
Abstract
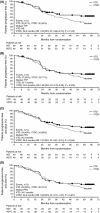
This follow-up extension of a randomised phase II study assessed differences in long-term outcomes between bortezomib-thalidomide-dexamethasone (VTD) and VTD-cyclophosphamide (VTDC) induction therapy in multiple myeloma. Newly diagnosed patients (n = 98) were randomised 1:1 to intravenous bortezomib (1·3 mg/m(2); days 1, 4, 8, 11), thalidomide (100 mg; days 1-21), and dexamethasone (40 mg; days 1-4, 9-12), with/without cyclophosphamide (400 mg/m(2); days 1, 8), for four 21-day cycles before stem-cell mobilisation/transplantation. After a median follow-up of 64·8 months, median time-to-next therapy was 51·8 and 47·9 months with VTD and VTDC, respectively. Type of subsequent therapy was similar in both arms. After adjusting for asymmetric censoring, median time to progression was not significantly different between VTD and VTDC [35·7 vs. 34·5 months; Hazard ratio (HR) 1·26, 95% confidence interval: 0·76-2·09; P = 0·370]. Five-year survival was 69·1% and 65·3% with VTD and VTDC, respectively. When analysed by minimal residual disease (MRD) status, overall survival was longer in MRD-negative versus MRD-positive patients with bone marrow-confirmed complete response (HR 3·66, P = 0·0318). VTD induction followed by transplantation provides long-term disease control and, consistent with the primary analysis, there is no additional benefit from adding cyclophosphamide. This study was registered at ClinicalTrials.gov (NCT00531453).
Keywords: minimal residual disease; multiple myeloma; transplantation.
© 2015 The Authors. British Journal of Haematology published by John Wiley & Sons Ltd.
Figures


References
-
- Anderson, K.C. , Alsina, M. , Bensinger, W. , Biermann, J.S. , Cohen, A.D. , Devine, S. , Djulbegovic, B. , Faber, E.A. Jr , Gasparetto, C. , Hernandez‐Illizaliturri, F. , Huff, C.A. , Kassim, A. , Krishnan, A.Y. , Liedtke, M. , Meredith, R. , Raje, N. , Schriber, J. , Singhal, S. , Somlo, G. , Stockerl‐Goldstein, K. , Treon, S.P. , Weber, D. , Yahalom, J. , Yunus, F. , Shead, D.A. & Kumar, R. (2013) Multiple myeloma, version 1.2013. Journal of the National Comprehensive Cancer Network, 11, 11–17. - PubMed
-
- Cavo, M. , Tacchetti, P. , Patriarca, F. , Petrucci, M.T. , Pantani, L. , Galli, M. , Di, R.F. , Crippa, C. , Zamagni, E. , Palumbo, A. , Offidani, M. , Corradini, P. , Narni, F. , Spadano, A. , Pescosta, N. , Deliliers, G.L. , Ledda, A. , Cellini, C. , Caravita, T. , Tosi, P. & Baccarani, M. (2010) Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. The Lancet, 376, 2075–2085. - PubMed
-
- Cavo, M. , Pantani, L. , Petrucci, M.T. , Patriarca, F. , Zamagni, E. , Donnarumma, D. , Crippa, C. , Boccadoro, M. , Perrone, G. , Falcone, A. , Nozzoli, C. , Zambello, R. , Masini, L. , Furlan, A. , Brioli, A. , Derudas, D. , Ballanti, S. , Dessanti, M.L. , de Stefano, V. , Carella, A.M. , Marcatti, M. , Nozza, A. , Ferrara, F. , Callea, V. , Califano, C. , Pezzi, A. , Baraldi, A. , Grasso, M. , Musto, P. & Palumbo A. (2012) Bortezomib‐thalidomide‐dexamethasone is superior to thalidomide‐dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood, 120, 9–19. - PubMed
-
- Cavo, M. , Galli, M. , Pezzi, A. , di Raimondo, F. , Crippa, C. , Offidani, M. , Tacchetti, P. , Montefusco, V. , Narni, F. , Spadano, A. , Pescosta, N. , Zamagni, E. , Gamberi, B. , Caravita, T. , Falcone, A.P. , Nozzoli, C. , Zambello, R. , Furlan, A. , Brioli, A. & Boccadaro, M. (2013) Persistent improvement in clinical outcomes with bortezomib‐thalidomide‐dexamethasone vs thalidomide‐dexamethasone incorporated into double autologous transplantation for multiple myeloma: an updated analysis of phase 3 GIMEMA‐MMY‐3006 study. Blood, 122, 2090.
-
- Cavo, M. , Pantani, L. , Pezzi, A. , Cavallo, F. , Petrucci, M.T. , Di Raimondo, F. , Patriarca, F. , Waage, A. , Zamagni, E. , Montefusco, V. , Galli, M. , Gamberi, B. , Rossi, G. , Tacchetti, P. , Grasso, M. , Zweegman, S. , Offidani, M. , Ballanti, S. , Zambello, R. , Liberati, A.M. , Bassan, R. , Pregno, P. , Palumbo, A. & Sonneveld, P. (2014) Superior efficacy of VTD over VCD as induction therapy for autotransplantation‐eligible, newly diagnosed, myeloma patients. Blood, 124, 197.

