A Method to Combine Signals from Spontaneous Reporting Systems and Observational Healthcare Data to Detect Adverse Drug Reactions
- PMID: 26153397
- PMCID: PMC4579260
- DOI: 10.1007/s40264-015-0314-8
A Method to Combine Signals from Spontaneous Reporting Systems and Observational Healthcare Data to Detect Adverse Drug Reactions
Abstract
Introduction: Observational healthcare data contain information useful for hastening detection of adverse drug reactions (ADRs) that may be missed by using data in spontaneous reporting systems (SRSs) alone. There are only several papers describing methods that integrate evidence from healthcare databases and SRSs. We propose a methodology that combines ADR signals from these two sources.
Objectives: The aim of this study was to investigate whether the proposed method would result in more accurate ADR detection than methods using SRSs or healthcare data alone.
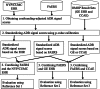
Research design: We applied the method to four clinically serious ADRs, and evaluated it using three experiments that involve combining an SRS with a single facility small-scale electronic health record (EHR), a larger scale network-based EHR, and a much larger scale healthcare claims database. The evaluation used a reference standard comprising 165 positive and 234 negative drug-ADR pairs.
Measures: Area under the receiver operator characteristics curve (AUC) was computed to measure performance.
Results: There was no improvement in the AUC when the SRS and small-scale HER were combined. The AUC of the combined SRS and large-scale EHR was 0.82 whereas it was 0.76 for each of the individual systems. Similarly, the AUC of the combined SRS and claims system was 0.82 whereas it was 0.76 and 0.78, respectively, for the individual systems.
Conclusions: The proposed method resulted in a significant improvement in the accuracy of ADR detection when the resources used for combining had sufficient amounts of data, demonstrating that the method could integrate evidence from multiple sources and serve as a tool in actual pharmacovigilance practice.
Figures


Comment in
-
Future Proofing Adverse Event Monitoring.Drug Saf. 2015 Oct;38(10):847-8. doi: 10.1007/s40264-015-0342-4. Drug Saf. 2015. PMID: 26323240 No abstract available.
References
-
- Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Washington, DC: 1996) 2001;41(2):192. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

