DAG tales: the multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling
- PMID: 26153463
- PMCID: PMC4575688
- DOI: 10.1007/s00018-015-1982-3
DAG tales: the multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling
Abstract
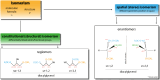
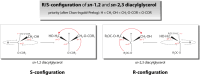
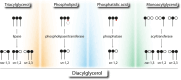
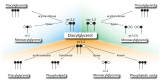
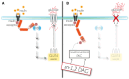
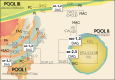
The neutral lipids diacylglycerols (DAGs) are involved in a plethora of metabolic pathways. They function as components of cellular membranes, as building blocks for glycero(phospho)lipids, and as lipid second messengers. Considering their central role in multiple metabolic processes and signaling pathways, cellular DAG levels require a tight regulation to ensure a constant and controlled availability. Interestingly, DAG species are versatile in their chemical structure. Besides the different fatty acid species esterified to the glycerol backbone, DAGs can occur in three different stereo/regioisoforms, each with unique biological properties. Recent scientific advances have revealed that DAG metabolizing enzymes generate and distinguish different DAG isoforms, and that only one DAG isoform holds signaling properties. Herein, we review the current knowledge of DAG stereochemistry and their impact on cellular metabolism and signaling. Further, we describe intracellular DAG turnover and its stereochemistry in a 3-pool model to illustrate the spatial and stereochemical separation and hereby the diversity of cellular DAG metabolism.
Keywords: Acyltransferase; Hydrolase; Insulin; Kinase; Lipase.
Figures
Similar articles
-
DIP2 is a unique regulator of diacylglycerol lipid homeostasis in eukaryotes.Elife. 2022 Jun 29;11:e77665. doi: 10.7554/eLife.77665. Elife. 2022. PMID: 35766356 Free PMC article.
-
Structure and functional properties of diacylglycerols in membranes.Prog Lipid Res. 1999 Jan;38(1):1-48. doi: 10.1016/s0163-7827(98)00021-6. Prog Lipid Res. 1999. PMID: 10396601 Review.
-
Signal transduction in vascular smooth muscle: diacylglycerol second messengers and PKC action.Am J Physiol. 1994 Sep;267(3 Pt 1):C659-78. doi: 10.1152/ajpcell.1994.267.3.C659. Am J Physiol. 1994. PMID: 7943196 Review.
-
Diacylglycerol generated in CHO cell plasma membrane by phospholipase C is used for triacylglycerol synthesis.J Lipid Res. 2001 Jan;42(1):88-95. J Lipid Res. 2001. PMID: 11160369
-
Functions of diacylglycerol in glycerolipid metabolism, signal transduction and cellular transformation.Oncogene Res. 1988 Feb;2(3):205-18. Oncogene Res. 1988. PMID: 3285300 Review.
Cited by
-
Effects of Fish Oil and Grape Seed Extract Combination on Hepatic Endogenous Antioxidants and Bioactive Lipids in Diet-Induced Early Stages of Insulin Resistance in Rats.Mar Drugs. 2020 Jun 16;18(6):318. doi: 10.3390/md18060318. Mar Drugs. 2020. PMID: 32560216 Free PMC article.
-
Obesity leads to distinct metabolomic signatures in follicular fluid of women undergoing in vitro fertilization.Am J Physiol Endocrinol Metab. 2019 Mar 1;316(3):E383-E396. doi: 10.1152/ajpendo.00401.2018. Epub 2019 Jan 2. Am J Physiol Endocrinol Metab. 2019. PMID: 30601701 Free PMC article.
-
Faecal Proteomics and Functional Analysis of Equine Melanocytic Neoplasm in Grey Horses.Vet Sci. 2022 Feb 21;9(2):94. doi: 10.3390/vetsci9020094. Vet Sci. 2022. PMID: 35202347 Free PMC article.
-
Hepatic Hedgehog Signaling Participates in the Crosstalk between Liver and Adipose Tissue in Mice by Regulating FGF21.Cells. 2022 May 18;11(10):1680. doi: 10.3390/cells11101680. Cells. 2022. PMID: 35626717 Free PMC article.
-
Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: a review.Lipids Health Dis. 2020 May 28;19(1):113. doi: 10.1186/s12944-020-01286-8. Lipids Health Dis. 2020. PMID: 32466765 Free PMC article. Review.
References
-
- Prelog V, Helmchen G. Basic principles of the CIP system and proposals for a revision. Angew Chemie, Int Ed english. 1982;21:567–583. doi: 10.1002/anie.198205671. - DOI
-
- Cahn RS, Ingold C, Prelog V. Specifications of molecular chirality. Angew Chemie Int Ed english. 1966;5:385–415. doi: 10.1002/anie.196603851. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

