Hydrophobic surfactant proteins strongly induce negative curvature
- PMID: 26153706
- PMCID: PMC4571016
- DOI: 10.1016/j.bpj.2015.05.030
Hydrophobic surfactant proteins strongly induce negative curvature
Abstract
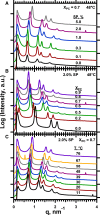
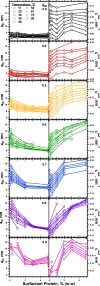
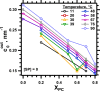
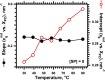
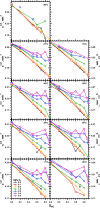
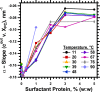
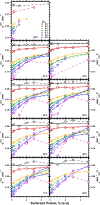
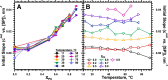
The hydrophobic surfactant proteins SP-B and SP-C greatly accelerate the adsorption of vesicles containing the surfactant lipids to form a film that lowers the surface tension of the air/water interface in the lungs. Pulmonary surfactant enters the interface by a process analogous to the fusion of two vesicles. As with fusion, several factors affect adsorption according to how they alter the curvature of lipid leaflets, suggesting that adsorption proceeds via a rate-limiting structure with negative curvature, in which the hydrophilic face of the phospholipid leaflets is concave. In the studies reported here, we tested whether the surfactant proteins might promote adsorption by inducing lipids to adopt a more negative curvature, closer to the configuration of the hypothetical intermediate. Our experiments used x-ray diffraction to determine how the proteins in their physiological ratio affect the radius of cylindrical monolayers in the negatively curved, inverse hexagonal phase. With binary mixtures of dioleoylphosphatidylethanolamine (DOPE) and dioleoylphosphatidylcholine (DOPC), the proteins produced a dose-related effect on curvature that depended on the phospholipid composition. With DOPE alone, the proteins produced no change. With an increasing mol fraction of DOPC, the response to the proteins increased, reaching a maximum 50% reduction in cylindrical radius at 5% (w/w) protein. This change represented a doubling of curvature at the outer cylindrical surface. The change in spontaneous curvature, defined at approximately the level of the glycerol group, would be greater. Analysis of the results in terms of a Langmuir model for binding to a surface suggests that the effect of the lipids is consistent with a change in the maximum binding capacity. Our findings show that surfactant proteins can promote negative curvature, and support the possibility that they facilitate adsorption by that mechanism.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Zimmerberg J., Kozlov M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006;7:9–19. - PubMed
-
- Markin V.S., Kozlov M.M., Borovjagin V.L. On the theory of membrane fusion. The stalk mechanism. Gen. Physiol. Biophys. 1984;3:361–377. - PubMed
-
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. C. 1973;28:693–703. - PubMed
-
- Tamm L.K., Han X. Viral fusion peptides: a tool set to disrupt and connect biological membranes. Biosci. Rep. 2000;20:501–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

