Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- PMID: 26153886
- PMCID: PMC4495997
- DOI: 10.1371/journal.ppat.1005028
Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
Abstract
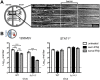
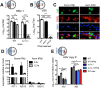
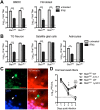
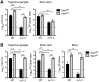
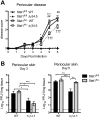
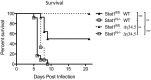
Interferon (IFN) responses are critical for controlling herpes simplex virus 1 (HSV-1). The importance of neuronal IFN signaling in controlling acute and latent HSV-1 infection remains unclear. Compartmentalized neuron cultures revealed that mature sensory neurons respond to IFNβ at both the axon and cell body through distinct mechanisms, resulting in control of HSV-1. Mice specifically lacking neural IFN signaling succumbed rapidly to HSV-1 corneal infection, demonstrating that IFN responses of the immune system and non-neuronal tissues are insufficient to confer survival following virus challenge. Furthermore, neurovirulence was restored to an HSV strain lacking the IFN-modulating gene, γ34.5, despite its expected attenuation in peripheral tissues. These studies define a crucial role for neuronal IFN signaling for protection against HSV-1 pathogenesis and replication, and they provide a novel framework to enhance our understanding of the interface between host innate immunity and neurotropic pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Blyth WA, Harbour DA, Hill TJ. Pathogenesis of zosteriform spread of herpes simplex virus in the mouse. J Gen Virol. 1984;65 (Pt 9): 1477–1486. - PubMed
-
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA J Am Med Assoc. 2006;296: 964–973. - PubMed
-
- Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359: 507–513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

