Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth
- PMID: 26154068
- PMCID: PMC4652186
- DOI: 10.1089/scd.2015.0104
Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth
Abstract
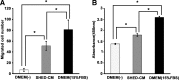
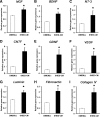
Peripheral nerve regeneration across nerve gaps is often suboptimal, with poor functional recovery. Stem cell transplantation-based regenerative therapy is a promising approach for axon regeneration and functional recovery of peripheral nerve injury; however, the mechanisms remain controversial and unclear. Recent studies suggest that transplanted stem cells promote tissue regeneration through a paracrine mechanism. We investigated the effects of conditioned media derived from stem cells from human exfoliated deciduous teeth (SHED-CM) on peripheral nerve regeneration. In vitro, SHED-CM-treated Schwann cells exhibited significantly increased proliferation, migration, and the expression of neuron-, extracellular matrix (ECM)-, and angiogenesis-related genes. SHED-CM stimulated neuritogenesis of dorsal root ganglia and increased cell viability. Similarly, SHED-CM enhanced tube formation in an angiogenesis assay. In vivo, a 10-mm rat sciatic nerve gap model was bridged by silicon conduits containing SHED-CM or serum-free Dulbecco's modified Eagle's medium. Light and electron microscopy confirmed that the number of myelinated axons and axon-to-fiber ratio (G-ratio) were significantly higher in the SHED-CM group at 12 weeks after nerve transection surgery. The sciatic functional index (SFI) and gastrocnemius (target muscle) wet weight ratio demonstrated functional recovery. Increased compound muscle action potentials and increased SFI in the SHED-CM group suggested sciatic nerve reinnervation of the target muscle and improved functional recovery. We also observed reduced muscle atrophy in the SHED-CM group. Thus, SHEDs may secrete various trophic factors that enhance peripheral nerve regeneration through multiple mechanisms. SHED-CM may therefore provide a novel therapy that creates a more desirable extracellular microenvironment for peripheral nerve regeneration.
Figures






References
-
- Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE. and Cullen DK. (2011). Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng 39:81–124 - PubMed
-
- Pabari A, Yang SY, Seifalian AM. and Mosahebi A. (2010). Modern surgical management of peripheral nerve gap. J Plast Reconstr Aesthet Surg 63:1941–1948 - PubMed
-
- Tang S, Zhu J, Xu Y, Xiang AP, Jiang MH. and Quan D. (2013). The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials 34:7086–7096 - PubMed
-
- Lin MY, Manzano G. and Gupta R. (2013). Nerve allografts and conduits in peripheral nerve repair. Hand Clin 29:331–348 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

