A novel TXNIP-based mechanism for Cx43-mediated regulation of oxidative drug injury
- PMID: 26154105
- PMCID: PMC4594688
- DOI: 10.1111/jcmm.12641
A novel TXNIP-based mechanism for Cx43-mediated regulation of oxidative drug injury
Abstract
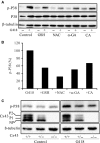
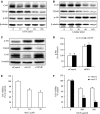
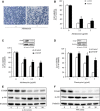
Gap junctions (GJs) play an important role in the regulation of cell response to many drugs. However, little is known about their mechanisms. Using an in vitro model of cytotoxicity induced by geneticin (G418), we explored the potential signalling mechanisms involved. Incubation of cells with G418 resulted in cell death, as indicated by the change in cell morphology, loss of cell viability and activation of caspase-3. Before the onset of cell injury, G418 induced reactive oxygen species (ROS) generation, activated oxidative sensitive kinase P38 and caused a shift of connexin 43 (Cx43) from non-phosphorylated form to hyperphosphorylated form. These changes were largely prevented by antioxidants, suggesting an implication of oxidative stress. Downregulation of Cx43 with inhibitors or siRNA suppressed the expression of thioredoxin-interacting protein (TXNIP), activated Akt and protected cells against the toxicity of G418. Further analysis revealed that inhibition of TXNIP with siRNA activated Akt and reproduced the protective effect of Cx43-inhibiting agents, whereas suppression of Akt sensitized cells to the toxicity of G418. Furthermore, interference of TXNIP/Akt also affected puromycin- and adriamycin-induced cell injury. Our study thus characterized TXNIP as a presently unrecognized molecule implicated in the regulatory actions of Cx43 on oxidative drug injury. Targeting Cx43/TXNIP/Akt signalling cascade might be a promising approach to modulate cell response to drugs.
Keywords: Akt; TXNIP; connexin 43; cytotoxicity; gap junction; oxidative stress.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



Similar articles
-
Disruption of gap junctions attenuates aminoglycoside-elicited renal tubular cell injury.Br J Pharmacol. 2010 Aug;160(8):2055-68. doi: 10.1111/j.1476-5381.2010.00860.x. Br J Pharmacol. 2010. PMID: 20649601 Free PMC article.
-
The pivotal role of extracellular signal-regulated kinase in gap junction-mediated regulation of TXNIP.Cell Signal. 2017 Oct;38:116-126. doi: 10.1016/j.cellsig.2017.07.001. Epub 2017 Jul 8. Cell Signal. 2017. PMID: 28694028
-
Gap junctions amplify TRPV4 activation-initiated cell injury via modification of intracellular Ca2+ and Ca2+-dependent regulation of TXNIP.Channels (Austin). 2020 Dec;14(1):246-256. doi: 10.1080/19336950.2020.1803552. Channels (Austin). 2020. PMID: 32752916 Free PMC article.
-
Involvement of Cx43 phosphorylation in 5'-N-ethylcarboxamide-induced migration and proliferation of mouse embryonic stem cells.J Cell Physiol. 2010 Jul;224(1):187-94. doi: 10.1002/jcp.22117. J Cell Physiol. 2010. PMID: 20232318
-
A role of connexin 43 on the drug-induced liver, kidney, and gastrointestinal tract toxicity with associated signaling pathways.Life Sci. 2021 Sep 1;280:119629. doi: 10.1016/j.lfs.2021.119629. Epub 2021 May 15. Life Sci. 2021. PMID: 34004253 Review.
Cited by
-
Connexins and Glucose Metabolism in Cancer.Int J Mol Sci. 2022 Sep 5;23(17):10172. doi: 10.3390/ijms231710172. Int J Mol Sci. 2022. PMID: 36077565 Free PMC article. Review.
-
Bioglass promotes wound healing by inhibiting endothelial cell pyroptosis through regulation of the connexin 43/reactive oxygen species (ROS) signaling pathway.Lab Invest. 2022 Jan;102(1):90-101. doi: 10.1038/s41374-021-00675-6. Epub 2021 Sep 14. Lab Invest. 2022. PMID: 34521991
-
DNA topoisomerase IIIβ promotes cyst generation by inducing cyst wall protein gene expression in Giardia lamblia.Open Biol. 2020 Feb;10(2):190228. doi: 10.1098/rsob.190228. Epub 2020 Feb 5. Open Biol. 2020. PMID: 32019477 Free PMC article.
-
A Novel Multiprotein Bridging Factor 1-Like Protein Induces Cyst Wall Protein Gene Expression and Cyst Differentiation in Giardia lamblia.Int J Mol Sci. 2021 Jan 29;22(3):1370. doi: 10.3390/ijms22031370. Int J Mol Sci. 2021. PMID: 33573049 Free PMC article.
-
A myeloid leukemia factor homolog is involved in tolerance to stresses and stress-induced protein metabolism in Giardia lamblia.Biol Direct. 2023 Apr 24;18(1):20. doi: 10.1186/s13062-023-00378-6. Biol Direct. 2023. PMID: 37095576 Free PMC article.
References
-
- Saez JC, Berthoud VM, Branes MC, et al. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–400. - PubMed
-
- Yao J, Oite T, Kitamura M. Gap junctional intercellular communication in the juxtaglomerular apparatus. Am J Physiol Renal Physiol. 2009;296:F939–46. - PubMed
-
- Loewenstein WR, Kanno Y. Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature. 1966;209:1248–9. - PubMed
-
- Yotti LP, Chang CC, Trosko JE. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science. 1979;206:1089–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

