The Effects of the Recombinant CCR5 T4 Lysozyme Fusion Protein on HIV-1 Infection
- PMID: 26154172
- PMCID: PMC4496087
- DOI: 10.1371/journal.pone.0131894
The Effects of the Recombinant CCR5 T4 Lysozyme Fusion Protein on HIV-1 Infection
Abstract
Background: Insertion of T4 lysozyme (T4L) into the GPCR successfully enhanced GPCR protein stability and solubilization. However, the biological functions of the recombinant GPCR protein have not been analyzed.
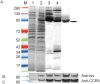
Methods: We engineered the CCR5-T4L mutant and expressed and purified the soluble recombinant protein using an E.coli expression system. The antiviral effects of this recombinant protein in THP-1 cell lines, primary human macrophages, and PBMCs from different donors were investigated. We also explored the possible mechanisms underlying the observed antiviral effects.
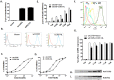
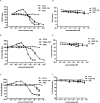
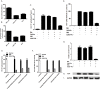
Results: Our data showed the biphasic inhibitory and promotion effects of different concentrations of soluble recombinant CCR5-T4L protein on R5 tropic human immunodeficiency virus-1 (HIV-1) infection in THP-1 cell lines, human macrophages, and PBMCs from clinical isolates. We demonstrated that soluble recombinant CCR5-T4L acts as a HIV-1 co-receptor, interacts with wild type CCR5, down-regulates the surface CCR5 expression in human macrophages, and interacts with CCL5 to inhibit macrophage migration. Using binding assays, we further determined that recombinant CCR5-T4L and [125I]-CCL5 compete for the same binding site on wild type CCR5.
Conclusions: Our results suggest that recombinant CCR5-T4L protein marginally promotes HIV-1 infection at low concentrations and markedly inhibits infection at higher concentrations. This recombinant protein may be helpful in the future development of anti-HIV-1 therapeutic agents.
Conflict of interest statement
Figures
Similar articles
-
Inhibition of CCR5-mediated infection by diverse R5 and R5X4 HIV and SIV isolates using novel small molecule inhibitors of CCR5: effects of viral diversity, target cell and receptor density.Antiviral Res. 2005 Nov;68(2):96-108. doi: 10.1016/j.antiviral.2005.07.006. Epub 2005 Aug 30. Antiviral Res. 2005. PMID: 16157392
-
Hemofiltrate CC chemokine 1[9-74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages.Antimicrob Agents Chemother. 2002 Apr;46(4):982-90. doi: 10.1128/AAC.46.4.982-990.2002. Antimicrob Agents Chemother. 2002. PMID: 11897579 Free PMC article.
-
The LD78beta isoform of MIP-1alpha is the most potent CC-chemokine in inhibiting CCR5-dependent human immunodeficiency virus type 1 replication in human macrophages.J Virol. 2001 May;75(9):4402-6. doi: 10.1128/JVI.75.9.4402-4406.2001. J Virol. 2001. PMID: 11287590 Free PMC article.
-
Therapeutic approaches to human immunodeficiency virus: structural studies on G-protein-coupled receptors.Pharmacol Ther. 1997 Oct-Dec;76(1-3):135-9. doi: 10.1016/s0163-7258(97)00109-5. Pharmacol Ther. 1997. PMID: 9535175 Review.
-
[Role of cellular proteins in the life cycle of human immunodeficiency virus].Vopr Virusol. 2009 Jan-Feb;54(1):4-7. Vopr Virusol. 2009. PMID: 19253722 Review. Russian.
Cited by
-
Lysozyme: A Natural Product with Multiple and Useful Antiviral Properties.Molecules. 2024 Jan 30;29(3):652. doi: 10.3390/molecules29030652. Molecules. 2024. PMID: 38338396 Free PMC article. Review.
-
Antivirals against animal viruses.Biochem Pharmacol. 2017 Jun 1;133:97-116. doi: 10.1016/j.bcp.2016.09.029. Epub 2016 Sep 30. Biochem Pharmacol. 2017. PMID: 27697545 Free PMC article. Review.
-
Lysozyme-Antimicrobial Peptide Fusion Protein Promotes the Diabetic Wound Size Reduction in Streptozotocin (STZ)-Induced Diabetic Rats.Med Sci Monit. 2018 Nov 23;24:8449-8458. doi: 10.12659/MSM.912596. Med Sci Monit. 2018. PMID: 30468157 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

